Nyob rau hauv geology thiab lub cev geography, ib tug toj siab (/pl?ˈto?/, /plæˈto?/, los yog /ˈplæto?/; French: [pla.to]; plural plateaus los yog plateaux), kuj hu ua ib tug siab plain los yog ib tug tableland, yog thaj chaw ntawm thaj chaw siab, feem ntau suav nrog cov av tiaj tus, uas tau nce siab tshaj qhov chaw ib puag ncig, feem ntau nrog ib lossis. Kawg hloov kho: 2023-12-15 23:12
Qee lub hauv paus muaj xws li: Ib lub xov tooj-xws li lub xov tooj tshwj xeeb lossis xov tooj ntawm tes. Cov dej haus (thiab cov thawv ntim cov kua txiv hmab txiv ntoo, tshwj xeeb tshaj yog cov menyuam yaus yuav nyob ntawd) Cov khoom noj xws li cov khoom noj uas khaws cia, cov qhob noom xim kasfes, MREs, cov kaus poom me me ntawm cov txiv hmab txiv ntoo qhuav thiab zaub. Portable chav dej, ntawv tso quav tso quav, thiab moistened wipes. Kawg hloov kho: 2023-12-15 23:12
Tsim cov kab mob hloov pauv caj ces (GMO) yog txheej txheem ntau kauj ruam. Genetic engineers yuav tsum cais cov noob uas lawv xav kom ntxig rau hauv lub cev. Cov noob no tuaj yeem raug coj los ntawm ib lub xovtooj ntawm tes lossis cov khoom siv hluavtaws. Kawg hloov kho: 2023-12-15 23:12
1.2 Cell Chemistry. Lub roj teeb nickel-cadmium muaj cov nickel-positive electrode (cathode) thiab cadmium-tsis zoo electrode (anode) hauv cov tshuaj potassium hydroxide. Nrog them, thermodynamically instable npib tsib xee (III)-hydroxide thiab siab dua hydroxides yog tsim los ntawm protonation ntawm nickel (II)-hydroxide. Kawg hloov kho: 2023-12-15 23:12
Cov hauv qab no yog cov yam ntxwv tseem ceeb ntawm hav zoov biome: qhov loj tshaj plaws thiab feem ntau nyuaj terrestrial biome. dominated ntoo thiab lwm yam ntoo ntoo. Lub luag haujlwm tseem ceeb hauv ntiaj teb kev noj cov pa roj carbon dioxide thiab tsim cov pa oxygen. raug hem los ntawm deforestation rau txiav ntoo, ua liaj ua teb, thiab tib neeg nyob. Kawg hloov kho: 2023-12-15 23:12
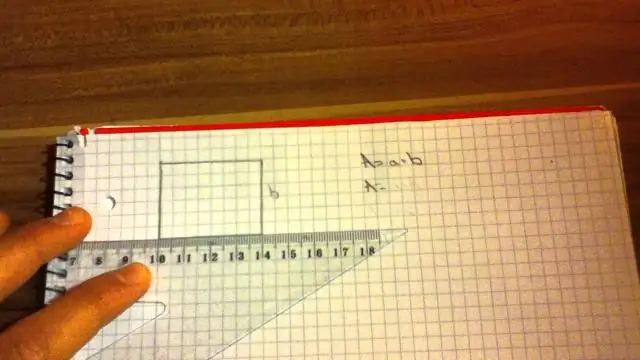
Thaum lub caij nplooj ntoos hlav txiav rau hauv ib nrab, nws yuav siv sij hawm ob zaug ntau zog kom ncav nws los ntawm qhov ntev. Tam sim no peb paub los ntawm kab zauv F = -kx tias k = -F / x qhov twg F yog lub zog yuav tsum tau ncab lub caij nplooj ntoos hlav los ntawm qhov deb x thiab k (Tag nrho seem muaj 135 lo lus.). Kawg hloov kho: 2023-12-15 23:12
Lesson Summary 'Thaum 'nce' yog xoom, ces kab yog kab rov tav, los yog tiaj tus, thiab txoj kab nqes hav yog xoom. Muab yooj yim, xoom txoj kab nqes yog zoo kawg nkaus tiaj tus nyob rau hauv txoj kab rov tav. Qhov sib npaug ntawm kab nrog xoom txoj kab nqes yuav tsis muaj x nyob rau hauv nws. Nws yuav zoo li 'y = ib yam dab tsi. Kawg hloov kho: 2023-12-15 23:12
Lub hauv qab-up mus kom ze yog lub piecing ua ke ntawm lub tshuab kom nce mus rau ntau complex systems, yog li ua rau cov thawj systems sub-systems ntawm lub tshwm sim system. Kev ua hauv qab yog ib hom kev ua cov ntaub ntawv raws li cov ntaub ntawv nkag los ntawm ib puag ncig los tsim kev nkag siab. Kawg hloov kho: 2023-12-15 23:12
Cov derivative tuaj yeem muab rau koj tus nqi ncaj qha rau qhov kev hloov pauv ntawd thiab ua rau tus qauv tsim nyog ntawm qhov xav tau kom muaj nuj nqis. Qhov tseem ceeb ntawm kev ua haujlwm tuaj yeem txhais geometrically raws li thaj tsam hauv qab ntawm qhov nkhaus ntawm kev ua lej f(x) plotted ua haujlwm ntawm x. Kawg hloov kho: 2023-12-15 23:12
Lub temperate hav zoov biome (Daim duab 2.3) npog kwv yees li ib feem tsib ntawm cov av muaj nyob rau hauv nruab nrab mus rau sab sauv latitudes thiab feem ntau nyob rau hauv cov cheeb tsam uas muaj ib tug zoo-txhais, tseem kuj me me, lub caij ntuj no. Kawg hloov kho: 2023-12-15 23:12
Lub atom hydrogen nrog lub zog ntau dhau tau hais tias "zoo siab". Ob txoj hauv kev los ua kom lub cev muaj zog yog los ntawm kev nqus lub teeb thiab los ntawm kev sib tsoo. Thaum ob lub atom sib tsoo lub zog sib pauv. Qee lub sij hawm, qee qhov ntawm lub zog yog siv los ua kom lub zog ntawm lub zog qis dua mus rau qib siab dua. Kawg hloov kho: 2023-12-15 23:12
Cov khoom siv organic. Organic cov ntaub ntawv tau txhais nyob rau hauv niaj hnub chemistry raws li carbon-raws li cov tebchaw, Ameslikas muab los ntawm cov kab mob nyob tab sis tam sim no suav nrog lab-synthesized versions thiab. [1] Feem ntau yog kev sib txuas ntawm ob peb lub teeb pom kev zoo, tshwj xeeb tshaj yog hydrogen, carbon, nitrogen, thiab oxygen. Kawg hloov kho: 2023-12-15 23:12
Ib qho piv txwv ntawm tib neeg ntau yam allele yog ABO hom ntshav, uas muaj peb hom alleles: IA, IB, thiab i. Piv txwv ntawm tib neeg cov yam ntxwv muaj xws li cov tawv nqaij xim thiab qhov siab ntawm cov neeg laus. Ntau yam zoo yog cuam tshuam los ntawm ib puag ncig, nrog rau cov noob. Qhov no tej zaum yuav muaj tseeb tshwj xeeb rau cov yam ntxwv polygenic. Kawg hloov kho: 2023-12-15 23:12
Teb thiab piav qhia: Cov kab mob no tsis suav tias yog cov cell nyob thiab yog li ntawd tsis yog ib leeg-celled lossis ntau-celled. Lawv tsuas yog suav hais tias yog cov plhaub protein. Kawg hloov kho: 2023-12-15 23:12
Cov zaub mov uas zoo ntawm cov zaub mov candida yog zaub ntsuab, nqaij, ntses, qe, zaub xam lav, almonds, walnuts, tshuaj ntsuab tshuaj yej, kua txiv hmab txiv ntoo, thiab cov txiv maj phaub dej tsis qab. Nrog rau kev noj zaub mov, nws qhia tias noj cov vitamins thiab probiotics, pw tsaug zog txaus, thiab zam kev ntxhov siab, uas txhua tus pab txhawb koj lub cev tiv thaiv kab mob. Kawg hloov kho: 2023-12-15 23:12
Kev loj hlob tus nqi tsob ntoo no loj hlob ntawm tus nqi nruab nrab, nrog qhov siab nce ntawm 13-24 'ib xyoos. Kawg hloov kho: 2023-12-15 23:12
Chemical Properties of Oxygen Ntawm tus qauv kub thiab siab (STP), ob lub atoms ntawm lub caij khi los tsim cov dioxygen, tsis muaj xim, tsis muaj ntxhiab, tsis muaj roj diatomic nrog cov mis O2. Oxygen yog ib tug tswv cuab ntawm pawg chalcogen nyob rau hauv lub sij hawm lub sij hawm thiab yog ib tug heev reactive nonmetallic keeb. Kawg hloov kho: 2023-12-15 23:12
Penetrance yog hais txog qhov feem pua ntawm cov neeg uas muaj kev hloov pauv caj ces (xws li kev hloov pauv hauv ib lub noob caj noob ces) uas pom cov cim thiab cov tsos mob ntawm cov kab mob caj ces. Yog tias qee tus neeg muaj kev hloov pauv tsis tau tsim cov yam ntxwv ntawm qhov tsis sib xws, qhov xwm txheej tau hais tias tau txo qis (lossis tsis tiav) nkag mus. Kawg hloov kho: 2023-12-15 23:12
Cov tshuaj tiv thaiv nrog barium nitrate: Ba(NO3)2 = Ba(NO2)2 + O2 (594-620 ° C), 2Ba(NO3)2 = 2BaO + 4NO2 + O2 (620-670 ° C). Ba(NO3)2 + 4H(0)(Zn, diluted HCl) = Ba(NO2)2 + 2H2O. Ba(NO3)2 + H2SO4(diluted) = BaSO4↓ + 2 HNO3. Kawg hloov kho: 2023-12-15 23:12
Hauv chemistry, lub zog tseem ceeb ntawm ib lub tshuab hluav taws xob yog hais txog lub plhaub lossis lub orbital uas cov hluav taws xob nyob ze rau lub atom lub nucleus. Qib no yog qhia los ntawm tus thawj xibfwb quantum n. Thawj lub caij nyob rau hauv ib lub sij hawm ntawm lub sij hawm lub sij hawm qhia ib tug tshiab tus thawj xib fwb theem zog. Kawg hloov kho: 2023-12-15 23:12
Harrison Cia li xav txog qhov no, leej twg yog Katherine tus thawj coj zais? Cam khwb cia (hauv kev txiav txim credits) ua tiav, tos kev pov thawj Taraji P. Henson Katherine G. Johnson Octavia Spencer Dorothy Vaughan Janelle Monáe Mary Jackson Kevin Costner Al Harrison Kirsten Dunst Vivian Mitchell Tom qab, lo lus nug yog, leej twg yog tus engineer hauv cov duab zais?. Kawg hloov kho: 2023-12-15 23:12
Cov tub hluas uas muaj XYY syndrome yuav muaj qee lossis tag nrho cov tsos mob ntawm lub cev mus rau qee qhov kev kawm: siab dua qhov siab nruab nrab. Cov leeg nqaij qis, lossis cov leeg tsis muaj zog (hu ua hypotonia) nkhaus heev pinky ntiv tes (hu ua clinodactyly). Kawg hloov kho: 2023-12-15 23:12
Lub zog tso tawm hauv cov tshuaj tiv thaiv fusion. Lub zog raug tso tawm nyob rau hauv cov tshuaj tiv thaiv nuclear yog tias tag nrho cov huab hwm coj ntawm cov khoom tshwm sim tsawg dua li qhov loj ntawm qhov pib reactants. Cov hais a thiab b feem ntau yog cov nucleons, xws li protons lossis neutrons, tab sis feem ntau tuaj yeem yog cov nuclei. Kawg hloov kho: 2023-12-15 23:12
Annus mirabilis cov ntaub ntawv. Kawg hloov kho: 2023-12-15 23:12
Manganese(II) Acetate Mn(C2H3O2)2 Molecular Luj - EndMemo. Kawg hloov kho: 2023-12-15 23:12
Cov kws saib xyuas mob niaj hnub siv qhov sib ntxiv, feem, qhov sib piv thiab qhov sib npaug ntawm algebraic txhua hnub ua haujlwm kom xa cov tshuaj zoo rau lawv cov neeg mob lossis saib xyuas kev hloov pauv hauv lawv txoj kev noj qab haus huv. Cov tsev kawm ntawv laus feem ntau sim cov tub ntxhais kawm tshiab ntawm lawv txoj kev ua lej, yuav tsum muaj kev kho mob hauv lej kho mob yog tias tsim nyog. Kawg hloov kho: 2023-12-15 23:12
Nuclear tshuaj tiv thaiv. Hauv nuclear physics, cov tshuaj tiv thaiv nuclear yog cov txheej txheem uas ob lub nuclei lossis nuclear hais sib tsoo, los tsim cov khoom sib txawv dua li cov khoom pib. Raws li txoj cai, cov tshuaj tiv thaiv tuaj yeem koom nrog ntau tshaj li ob qhov sib tsoo, tab sis qhov xwm txheej zoo li no tsis tshua muaj. Kawg hloov kho: 2023-12-15 23:12
Angular acceleration (α) tuaj yeem txhais tau tias yog angular tshaj tawm (ω) muab faib los ntawm lub sijhawm acceleration (t). Xwb, pi (π) multiplied by drive speed (n) muab faib los acceleration time (t) multiplied by 30. Qhov sib npaug no yields tus qauv angular acceleration SI unit ntawm radians ib ob squared (Rad/sec^2). Kawg hloov kho: 2023-12-15 23:12
Cov yam ntxwv: Tin yog xim dawb-dawb, mos, malleable hlau uas tuaj yeem ua polished heev. Tin muaj cov qauv crystalline heev thiab thaum lub tin bar yog khoov, lub 'tin quaj' tau hnov, vim yog tawg ntawm cov pob zeb no. Kawg hloov kho: 2023-12-15 23:12
Cov pab pawg ua haujlwm hydrophilic muaj xws li pawg hydroxyl (ua rau muaj cawv txawm tias tseem pom muaj suab thaj, thiab lwm yam), pawg carbonyl (ua rau aldehydes thiab ketones), pawg carboxyl (ua rau carboxylic acids), pawg amino (piv txwv li, raws li pom hauv cov amino acids. ), sulfhydryl pawg (ua rau thiols, piv txwv li, raws li pom. Kawg hloov kho: 2023-12-15 23:12
Lub teeb xiav yog tawg mus rau txhua qhov kev qhia los ntawm cov me me molecules ntawm huab cua hauv ntiaj teb huab cua. Xiav tawg ntau dua li lwm cov xim vim tias nws taug kev asshorer, me nthwv dej. Qhov no yog vim li cas peb pom ib tug xiav skymost ntawm lub sij hawm. Kaw rau lub qab ntug, lub ntuj ploj mus alighter xiav los yog dawb. Kawg hloov kho: 2023-12-15 23:12
Cov pH ntawm Polar Desert av sib txawv los ntawm kwv yees li 4.4 mus txog 7.9. Ib yam li cov hluav taws xob conductivity ntawm tsawg dua 10 txog 66 mΩ cm 1. Kawg hloov kho: 2023-12-15 23:12
NASA cov neeg ua haujlwm khwv tau $ 63,500 ib xyoos ib zaug, lossis $ 31 toj ib teev, uas yog 2% siab dua li cov nyiaj hli nruab nrab ntawm $ 62,000 toj xyoo. Raws li peb cov ntaub ntawv, txoj haujlwm them nyiaj siab tshaj plaws ntawm NASA yog Lead Engineerat $ 126,000 ib xyoos ib zaug thaum lub luag haujlwm them qis tshaj ntawm NASA isa Student Researcher ntawm $ 21,000 txhua xyoo. Kawg hloov kho: 2023-12-15 23:12
Qhov tseem ceeb tshaj plaws yog tus naj npawb ntawm cov ions tsis zoo uas sib npaug rau tus nqi ntawm cov hlau ion. Qhov thib ob valency yog tus naj npawb ntawm ligands uas txuas los yog sib koom ua ke rau hlau ion. Kawg hloov kho: 2023-12-15 23:12
Cov khoom siv hluav taws xob & hluav taws xob cov lus Lub Npe Lub Npe Lub Npe Lub Npe Ampere (amp) Ib qho hluav taws xob tam sim no (I) Volt V Voltage (V, E) Electromotive force (E) Muaj peev xwm sib txawv (Δφ) Ohm Ω Resistance (R) Watt W Fais fab mov (P). Kawg hloov kho: 2023-12-15 23:12
Ntau tus neeg kawm tiav mus rau kev ua haujlwm xws li PE cov kws qhia ntawv, kws qhia kis las, tus kws qhia qoj thiab tus kws qhia tus kheej, txawm hais tias kev tswj hwm, kev koom tes thiab kev txhawb zog uas yog lub hauv paus rau feem ntau cov kev kawm txuj ci kis las pub rau cov neeg kawm tiav kev ua kis las kom hloov mus rau ntau lub luag haujlwm. Kawg hloov kho: 2023-12-15 23:12
Cov hnub qub tau yug los thaum huab cua loj tawg hauv qab lub ntiajteb txawj nqus. Thaum nws kawg tuag, nws yuav nthuav mus rau ib daim ntawv hu ua 'liab loj heev' thiab tom qab ntawd tag nrho cov txheej txheej ntawm lub hnub yuav maj mam tshuab tawm mus rau hauv qhov chaw tawm tsuas yog lub hnub qub me me Dawb Dwarf qab txog qhov loj ntawm lub ntiaj teb. Kawg hloov kho: 2023-12-15 23:12
Algebraic qhia ?: Cov lus lej suav nrog tsawg kawg ib qho sib txawv thiab qee zaum tus lej thiab cov cim ua haujlwm. Kawg hloov kho: 2023-12-15 23:12
Tus lej khib ntawm cov ntsiab lus zoo dua ntawm kev ntes koj qhov ntsia. Cov lej khib yuam kom koj lub qhov muag txav mus los ntawm kev sib koom ua ke - thiab los ntawm kev txuas ntxiv, chav. Qhov kev quab yuam quab yuam yog lub plawv ntawm qhov pom kev txaus siab. Nws yog vim li ntawd hais tias ib pawg ntawm peb yog qhov txaus siab thiab nco ntau dua li ib yam dab tsi ua khub hauv ob lub. Kawg hloov kho: 2023-12-15 23:12
Silver sulfate Lub npe Melting point 652.2–660 °C (1,206.0–1,220.0 °F; 925.4–933.1 K) Boiling point 1,085 °C (1,985 °F; 1,358 K) Solubility hauv dej 0.57 g / 100 ° C / 100 mL (10 ° C) 0.83 g / 100 mL (25 ° C) 0.96 g / 100 mL (40 ° C) 1.33 g / 100 mL (100 ° C) Solubility khoom (Ksp) 1.2 · 10− 5. Kawg hloov kho: 2023-12-15 23:12