
Video: Muaj pes tsawg daim ntawv cog lus sulfur?

2024 Tus sau: Miles Stephen | [email protected]. Kawg hloov kho: 2023-12-15 23:36
Sulfur feem ntau tsim 2 daim ntawv cog lus , np. H2S, -S-S-compounds Qhov no yog vim nws 3p4 orbital. p-orbitals tso cai rau 6 qhov chaw kom puv, li no sulfur nyhav tsim 2 daim ntawv cog lus . Nws tuaj yeem "nthuav cov octet" raws li nws muaj 6 valence electrons, ua rau muaj kev tsim ntawm 6 daim ntawv cog lus.
Ib yam li ntawd, tej zaum koj yuav nug tias, Sulfur muaj pes tsawg daim ntawv cog lus?
ob daim ntawv cog lus
Tsis tas li ntawd, sulfur puas tuaj yeem muaj 4 daim ntawv cog lus? Phosphorus tuaj yeem tsim mus txog tsib covalent daim ntawv cog lus , raws li nyob rau hauv phosphoric acid (H3PO 4 ). Feem ntau vim nws qhov chaw sab hnub tuaj tshaj plaws yog qhov loj dua li cov pa oxygen, sulfur canform tsawg li ob covalent daim ntawv cog lus , zoo li hauv hydrogensulfide (H2S), los yog ntau li rau, xws li hauv leej faj trioxide (SO3los yog sulfuric acid (H2YOG 4 ):
Tib neeg kuj nug, leej twg puas muaj 6 daim ntawv cog lus?
Teb thiab piav qhia: Sulfur muaj peev xwm ua tau 6 daim ntawv cog lus vim nws tuaj yeem muaj lub plhaub expandedvalence; leej faj yog nyob rau lub sijhawm 3 ntawm Lub Sijhawm Teev Taw.
Puas muaj leej faj ua tau triple bonds?
Tsiv electrons los ntawm ib leeg khub ntawm lub davhlau ya nyob twg atoms mus rau daim ntawv ob lub triple daim ntawv cog lus . Atoms uas nyiam daim ntawv ntau daim ntawv cog lus yog C, N, P, O, thiab S. Hydrogenand halogens TSIS TAU daim ntawv ob npaug daim ntawv cog lus . Qee cov molecules zoo li leej faj dioxide muaj qhov nthuav kev sib raug zoo xwm txheej.
Pom zoo:
Yuav ua li cas yog daim ntawv cog lus sib txawv ntawm daim ntawv cog lus ionic?
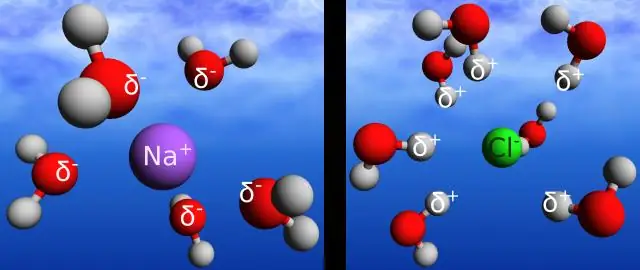
Qhov sib txawv ntawm cov ionic thiab covalent daim ntawv cog lus yog tias covalent daim ntawv cog lus yog tsim thaum ob lub atoms sib koom electrons. Ionic bonds yog lub zog uas tuav ua ke electrostatic zog ntawm kev nyiam ntawm cov ions oppositely them. Ionic bonds muaj qhov sib txawv electronegativity ntau dua lossis sib npaug li 2
Dab tsi yog qhov txawv ntawm daim ntawv cog lus lub zog thiab daim ntawv cog lus dissociation zog?
Qhov sib txawv tseem ceeb ntawm kev sib txuas ntawm lub zog thiab lub zog bonddissociation yog tias lub zog sib txuas yog qhov nruab nrab ntawm lub zog xav tau los rhuav tshem tag nrho cov kev sib txuas ntawm tib ob hom atoms nyob rau hauv ib qho chaw sib txuas whereas daim ntawv cog lus dissociation zog yog tus nqi ntawm cov hluav taws xob xav tau los rhuav tshem ib qho kev sib koom ua ke inhomolysis
Puas yog daim ntawv cog lus hydrogen zoo ib yam li daim ntawv cog lus?
Hydrogen daim ntawv cog lus yog lub npe muab rau electrostatic kev sib cuam tshuam ntawm tus nqi zoo ntawm hydrogen atom thiab tus nqi tsis zoo ntawm oxygen atom ntawm ib qho chaw nyob sib ze. Covalent daim ntawv cog lus yog qhov sib cuam tshuam electrostatic ntawm ob lub atom hauv tib lub molecule
Muaj pes tsawg daim ntawv cog lus tuaj yeem tsim carbon atom thiab vim li cas?

plaub Tsis tas li ntawd, vim li cas nws tseem ceeb uas cov pa roj carbon tsim 4 daim ntawv cog lus? Cov pa roj carbon yog tib lub ntsiab uas ua tau daim ntawv thiaj li sib txawv tebchaw vim txhua carbon atom tau daim ntawv plaub tshuaj daim ntawv cog lus rau lwm cov atoms, thiab vim cov carbon atom tsuas yog txoj cai, me me kom haum rau qhov yooj yim raws li qhov chaw ntawm cov molecules loj heev.
Muaj pes tsawg tus electrons raug muab faib rau hauv ob daim ntawv cog lus?

Hauv daim ntawv cog lus covalent ib khub ntawm electrons tau sib koom ntawm ob lub atoms 'txuas' los ntawm covalent daim ntawv cog lus. Yog li ib daim ntawv cog lus doublecovalent muaj ob khub ntawm electrons raug muab sib koom, yog li tag nrho plaub electrons
