Qhov ceev ntawm seawater (khoom siv) Seawater hnyav 1.024 gram ib cubic centimeter los yog 1 024 kilogram ib cubic meter, piv txwv li qhov ceev ntawm seawater yog sib npaug rau 1 024 kg / m³; ntawm 20 ° C (68 ° F lossis 293.15K) ntawm tus qauv atmospheric siab. Kawg hloov kho: 2023-12-15 23:12
Yam ntxwv: Gallium yog silvery, iav zoo li, mos hlau. Nws zaum ze rau cov tsis-hlau nyob rau hauv lub sij hawm lub rooj thiab nws cov khoom hlau tsis yog asobviously metallic li feem ntau lwm yam hlau. Solid gallium isbrittle thiab yog ib qho tsis zoo hluav taws xob conductor thanlead. Kawg hloov kho: 2023-12-15 23:12
Ob lub hlwb cog thiab tsiaj hlwb yog Eukaryotic hlwb. Cov no yog cov hlwb uas muaj cov nucleus uas muaj txiaj ntsig zoo thiab nyob rau hauv uas lwm cov organelles tau tuav ua ke los ntawm daim nyias nyias. Kawg hloov kho: 2023-12-15 23:12
Feem ntau, cov neeg txhawb nqa yog tsim los ntawm lub hauv paus ntsiab lus uas cov tshuab hloov pauv dav dav khi (piv txwv li, RNA polymerase II thiab cov TFs dav dav), thiab cov proximal gene proximal uas yog qhov chaw tsaws rau kev tswj hwm TFs. Kawg hloov kho: 2023-12-15 23:12
Ib coulomb ib ob. Kawg hloov kho: 2023-12-15 23:12
Qhov kawg ntawm interphase hu ua G2 theem. Lub cell tau loj hlob, DNA tau rov ua dua, thiab tam sim no lub xovtooj yuav luag npaj los faib. Qhov kawg theem no yog txhua yam hais txog kev npaj cov cell rau mitosis lossis meiosis. Thaum lub sij hawm G2, lub xov tooj ntawm tes yuav tsum loj hlob ib co ntxiv thiab tsim tej molecules nws tseem yuav tsum tau faib. Kawg hloov kho: 2023-12-15 23:12
Density of Elements Chart Density Name Symbol 0.862 g/cc Potassium K 0.971 g/cc Sodium Na 1.55 g/cc Calcium Ca 1.63 g/cc Rubidium Rb. Kawg hloov kho: 2023-12-15 23:12
Standard txoj hauj lwm ntawm lub kaum sab xis - trigonometry Ib sab ntawm lub kaum sab xis yog ib txwm kho raws li qhov zoo x-axis - uas yog, mus rau sab xis raws tus axis nyob rau hauv 3 teev kev taw qhia (kab BC). Qhov no yog hu ua thawj sab ntawm lub kaum sab xis. Lwm sab ntawm lub kaum sab xis yog hu ua lub davhlau ya nyob twg sab. Kawg hloov kho: 2023-12-15 23:12
1 Teb. Chloroplast thiab mitochondria tsis paub ua haujlwm ua ke. Txawm li cas los xij, cov piam thaj thiab oxygen tsim los ntawm photosynthesis nyob rau hauv chloroplasts yog xav tau los ntawm mitochondria thiaj li ua rau aerobic cellular respiration. Kawg hloov kho: 2023-12-15 23:12
Qhov Tseeb Txog Tennessine (Element 117) Tennessine yog lub xov tooj cua, tsim cov khoom tsim tawm uas tsis tshua paub. Nws yuav tsum yog ib qho khoom, tab sis nws qhov kev faib tawm tsis paub. Nws yog ib tug tswv cuab ntawm pawg halogen. Kawg hloov kho: 2023-12-15 23:12
Erosion cia siab rau kev thauj khoom xws li cua, dej ntws, dej khov, daus thiab nqes txav ntawm cov khoom siv los nqa cov khoom huab cua mus deb ntawm qhov chaw. Raws li huab cua cov khoom raug nqa mus, cov pob zeb tshiab tau raug rau huab cua ntxiv. Kawg hloov kho: 2023-12-15 23:12
Tshem tawm cov hluav taws xob los ntawm Protons Tshem tawm cov xov tooj ntawm cov hluav taws xob los ntawm cov xov tooj ntawm protons hauv ib lub atom raws li ib txoj hauv kev los xam cov nqi ntawm theion. Piv txwv li, yog hais tias ib tug sodium atom poob ib tug electron, workout 11 - 10 = 1. Ib tug sodium ion muaj ib tug +1 nqi, sau tseg asNa +. Kawg hloov kho: 2023-12-15 23:12
Pawg pob zeb Sedimentary ua kom tsawg tshaj plaws ntawm lub ntiaj teb crust nrog ib feem ntawm 8. Kawg hloov kho: 2023-12-15 23:12
Lub molecularity ntawm cov tshuaj tiv thaiv yog tus naj npawb ntawm cov molecules reacting nyob rau hauv ib theem pib. Ib qho tshuaj tiv thaiv unimolecular yog ib qho uas tsuas yog ib qho reacting molecule koom nrog cov tshuaj tiv thaiv. Ob lub reactant molecules sib tsoo nrog ib leeg hauv cov tshuaj tiv thaiv bimolecular. Kawg hloov kho: 2023-12-15 23:12
Txoj kev xav ntawm tes hais tias: - Txhua yam muaj sia nyob yog tsim los ntawm cov hlwb. Cov kab mob ntau lub cev (piv txwv: tib neeg) muaj ntau lub hlwb thaum cov kab mob unicellular (piv txwv: cov kab mob) tsuas yog tsim los ntawm ib lub xov tooj xwb. - Cells yog qhov tsawg tshaj plaws ntawm lub neej. Kawg hloov kho: 2023-12-15 23:12
Cim: Hester Prynne, Arthur Dimmesdale. Kawg hloov kho: 2023-12-15 23:12
Enzyme assay Enzyme assay yog txoj hauv kev kuaj rau kev ntsuas kev ua haujlwm enzymatic. Qhov kom muaj nuj nqis lossis concentration ntawm ib qho enzyme tuaj yeem qhia tau nyob rau hauv cov nyiaj molar, xws li nrog rau lwm yam tshuaj, lossis hais txog kev ua haujlwm hauv enzyme units. Kev ua enzyme = moles ntawm substrate hloov dua siab tshiab rau ib chav tsev lub sij hawm = tus nqi × cov tshuaj tiv thaiv ntim. Kawg hloov kho: 2024-01-18 08:01
Nyob rau hauv thaum ntxov 19th caug xyoo Jean-Baptiste Lamarck (1744-1829) tau npaj nws txoj kev xav ntawm kev hloov ntawm hom, thawj qhov kev xav ntawm evolution. Xyoo 1858 Charles Darwin thiab Alfred Russel Wallace tau luam tawm ib txoj kev xav tshiab evolutionary, piav qhia meej hauv Darwin's On the Origin of Species (1859). Kawg hloov kho: 2023-12-15 23:12
Spearman's Rho yog ib qho kev ntsuas uas tsis yog parametric siv los ntsuas lub zog ntawm kev sib koom ua ke ntawm ob qhov sib txawv, qhov twg tus nqi r = 1 txhais tau tias muaj kev sib raug zoo zoo meej thiab tus nqi r = -1 txhais tau hais tias kev sib raug zoo tsis zoo. Kawg hloov kho: 2023-12-15 23:12
Qhov kev sim Geiger-Marsden (tseem hu ua Rutherford kub foil sim) yog qhov kev sim ntawm qhov chaw uas cov kws tshawb fawb pom tau tias txhua lub atom muaj cov nucleus uas nws cov nqi zoo thiab feem ntau ntawm nws qhov loj yog concentrated. Kawg hloov kho: 2023-12-15 23:12
Cov tshuaj tiv thaiv tshuaj feem ntau cuam tshuam nrog kev hloov pauv ntawm lub zog vim kev tawg thiab tsim cov ntawv cog lus. Cov tshuaj tiv thaiv uas lub zog raug tso tawm yog cov tshuaj tiv thaiv exothermic, thaum cov uas siv lub zog cua sov yog endothermic. Kawg hloov kho: 2023-12-15 23:12
Lus Txhais ntawm Molecular Substances Nws yog ib yam khoom siv molecular, uas yog ib yam khoom uas muaj ob lossis ntau lub atoms, qhov tsawg tshaj plaws ntawm cov teeb meem, koom ua ke los ntawm covalent daim ntawv cog lus. Covalent daim ntawv cog lus yog qhov txuas tsim los ntawm kev sib koom ntawm cov hluav taws xob uas tuav cov atoms ua ke. Kawg hloov kho: 2023-12-15 23:12
Thaum kawg, tus nqi C yog +4. Cov calcium atom yog anelement pom nyob rau hauv pawg thib ob ntawm lub sij hawm. Cov ntsiab lus no poob ob lub tshuab hluav taws xob hauv cov tshuaj tiv thaiv tshuaj lossis muaj oxidation lub xeev 2+ hauv cov tshuaj lom neeg. Lwm txhais tes thecarbonate yog ib qho ion ntau hauv inorganic chemistry thiab muaj 2-them nqi. Kawg hloov kho: 2023-12-15 23:12
Pob zeb tumbling yog qhov kev nyiam los ntawm kev sau ntau cov pob zeb thiab tig lawv mus rau hauv cov pob zeb zoo nkauj uas koj tuaj yeem siv los ua cov hniav nyiaj hniav kub, khoom siv tes ua, dai kom zoo nkauj, lossis tsuas yog los sau rau kev lom zem. Txhua yam koj xav tau yog tumbler, qee pob zeb, thiab ob peb lwm yam khoom pheej yig. Kawg hloov kho: 2023-12-15 23:12
Evolutionism yog ib lo lus siv (feem ntau derogatorily) los qhia txog txoj kev xav ntawm evolution. Lub sij hawm yog tsis tshua muaj siv nyob rau hauv lub scientific zej zog, txij li thaum lub scientific txoj hauj lwm nyob rau hauv evolution tau txais los ntawm cov neeg coob coob ntawm cov kws tshawb fawb. Kawg hloov kho: 2023-12-15 23:12
Txoj cai lij choj ntawm kev txuag ntawm huab hwm coj hais tias huab hwm coj nyob rau hauv ib qho kev sib cais tsis yog tsim los yog rhuav tshem los ntawm cov tshuaj tiv thaiv lossis kev hloov ntawm lub cev. Raws li txoj cai lij choj ntawm kev txuag ntawm huab hwm coj, qhov loj ntawm cov khoom nyob rau hauv cov tshuaj tiv thaiv tshuaj yuav tsum sib npaug zos qhov loj ntawm cov reactants. Kawg hloov kho: 2023-12-15 23:12
Giant ionic qauv. Cov ions nyob rau hauv ib qho chaw, xws li sodium chloride, yog teem nyob rau hauv ib tug loj heev ionic qauv (tseem hu ua ib tug loj heev ionic lattice). Qhov no txhais tau hais tias ionic compounds muaj cov ntsiab lus melting siab thiab cov ntsiab lus kub. Cov khoom sib xyaw ionic tsis ua hluav taws xob vim tias cov ions tuav ruaj khov rau hauv qhov chaw. Kawg hloov kho: 2023-12-15 23:12
Nws yog Gram-zoo, pas nrig-puab thiab pom nrog lwm cov kab mob bacillus megaterium. Nws yog motile, nrog rau kev siv ntawm nws cov flagella. Lub cell phab ntsa, muaj ntau npaum li cas ntawm peptidoglycan. Kev khiav ntawm lub zog hauv kev ua pa ntawm tes yog suav tias yog aerobic, tab sis tuaj yeem raug mob anaerobic. Kawg hloov kho: 2023-12-15 23:12
Forceps. Siv los khaws lossis tuav cov khoom me me. Kawg hloov kho: 2023-12-15 23:12
Lub teeb pom kev ntawm Photosynthesis. Lub teeb yog absorbed thiab lub zog yog siv los tsav electrons los ntawm dej los tsim NADPH thiab tsav protons hla ib daim nyias nyias. Cov protons rov qab los ntawm ATP synthase los ua ATP. Kawg hloov kho: 2023-12-15 23:12
Teb thiab piav qhia: Ob tug qauv loj siv los ntawm cov pej xeem ecologists los ntsuas cov pej xeem kev loj hlob yog tus qauv kev loj hlob exponential thiab tus qauv kev loj hlob logistical. Kawg hloov kho: 2023-12-15 23:12
Lunar eclipse tshwm sim thaum lub ntiaj teb hla ntawm lub hli thiab lub hnub, thiab lub ntiaj teb tus duab ntxoov ntxoo obscures lub hli los yog ib feem ntawm nws. Hnub ci eclipse tshwm sim thaum lub hli hla ntawm lub ntiaj teb thiab lub hnub, thaiv tag nrho los yog ib feem ntawm lub hnub. Ib qho dab noj hnub tuaj yeem yog tag nrho, ib nrab, lossis annular. Kawg hloov kho: 2023-12-15 23:12
Covalent bonds tuaj yeem yog ib qho, ob npaug, thiab triple daim ntawv cog lus. Ib daim ntawv cog lus tshwm sim thaum ob lub tshuab hluav taws xob sib koom thiab yog tsim los ntawm ib daim ntawv cog lus ntawm ob lub atom. Ob daim ntawv cog lus tshwm sim thaum plaub lub tshuab hluav taws xob sib koom ntawm ob lub atom thiab muaj ib daim ntawv cog lus sigma thiab ib daim ntawv cog lus pi. Kawg hloov kho: 2023-12-15 23:12
Wave zaus ntsuas tau los ntawm kev suav cov crests (cov ntsiab lus siab) ntawm cov nthwv dej uas dhau qhov taw tes ruaj khov hauv 1 thib ob lossis qee lub sijhawm. Tus lej siab dua, qhov ntau dua ntawm cov nthwv dej. Kawg hloov kho: 2023-12-15 23:12
Ib qho kev hloov pauv hauv av yog qhov tseem ceeb zoo ib yam li cov hloov pauv hauv av, tab sis yog tsim los rau cov kev xav tau tshwj xeeb ntawm kev teeb tsa hauv av. Vault hom, ncoo-mounted, submersible, thiab ncaj-faus transformers yog siv nyob rau hauv underground systems. Kawg hloov kho: 2023-12-15 23:12
Qee cov lus feem ntau ntsib hauv analytical chemistry yog: qauv: theobject ntawm tus txheej txheem analytical (piv txwv li: ntshav kuaj); Analyte: cov khoom uas nyiam nyob rau hauv kev tsom xam (piv txwv li: tus nqi ntawm hemoglobin hauv cov ntshav);. Kawg hloov kho: 2023-12-15 23:12
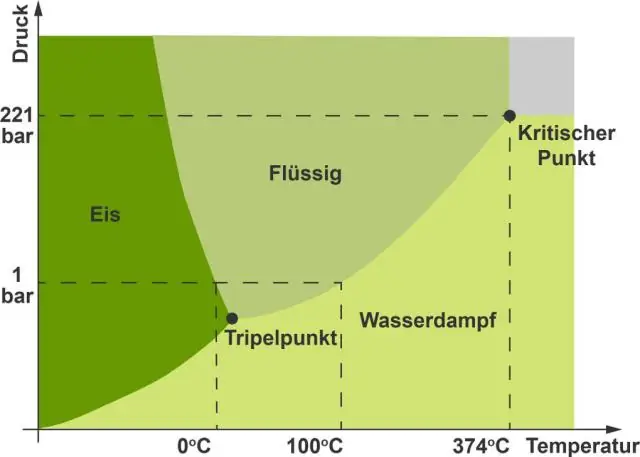
Hauv cov duab kos duab, txoj kab nqes ntawm txoj kab nruab nrab ntawm cov khoom thiab cov kua dej yog qhov tsis zoo es tsis zoo. Yog vim li cas yog tias dej yog ib yam khoom txawv txawv nyob rau hauv uas nws lub xeev yog tsawg ntom dua li cov kua hauv lub xeev. Kawg hloov kho: 2023-12-15 23:12
Cov imperial fasteners yog cov uas nws qhov ntev yog ntsuas siv imperial units ntawm kev ntsuas, thiab cov metrics yog cov uas ntsuas siv cov metric units. Kawg hloov kho: 2023-12-15 23:12
Evolution - Kev Kho Mob Txhais Kev Hloov pauv hauv cov caj ces muaj pes tsawg leeg ntawm cov pej xeem thaum lub sijhawm ua tiav, feem ntau ua rau kev loj hlob ntawm hom tshiab. Cov txheej txheem ntawm evolution muaj xws li kev xaiv ntuj tsim los ntawm kev hloov pauv caj ces ntawm tib neeg, kev hloov pauv, kev tsiv teb tsaws, thiab kev hloov caj ces. Kawg hloov kho: 2023-12-15 23:12
Nyob rau hauv methyl dawb radical lub hybridization yog sp2 vim hais tias nws muaj 3 daim ntawv cog lus khub thiab ib tug unpaired electron uas yog heev reactive yog li nyob rau hauv hybridization nws tsis suav nrog thiab 3 daim ntawv cog lus khub yog tam sim no ces ib tug mus nrog s thiab lwm yam 2 nrog p. Kawg hloov kho: 2023-12-15 23:12