Cov txheej txheem:
Video: Koj sau cov qauv condensed li cas?

2024 Tus sau: Miles Stephen | [email protected]. Kawg hloov kho: 2024-01-18 08:15
1 Teb
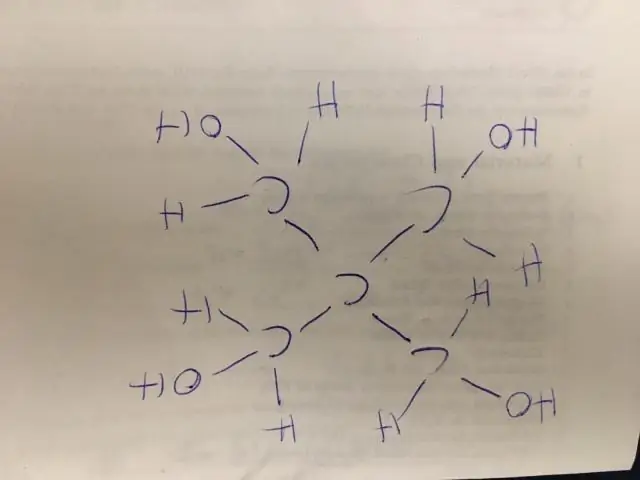
- Sau cov atoms ntawm cov saw ntev tshaj plaws horizontally nyob rau hauv qhov kev txiav txim nyob rau hauv uas lawv txuas nrog.
- Sau tag nrho cov ligands ntawm ib lub atom tam sim ntawd mus rau nws txoj cai, nrog subscripts rau ntau.
- Muab cov polyatomic ligands nyob rau hauv lub voj voog.
- Siv daim ntawv cog lus meej raws li xav tau los qhia meej cov ntawv txuas.
Dhau li ntawm no, dab tsi yog condensed structural formula muab piv txwv?
Nws qhia tag nrho cov atoms, tab sis tshem tawm cov ntawv cog lus ntsug thiab feem ntau lossis tag nrho cov kab rov tav ib daim ntawv cog lus. Nws siv cov kab lus los qhia tias pawg polyatomic nyob rau hauv ib qauv yog txuas rau qhov ze tshaj plaws uas tsis yog-hydrogen atom ntawm sab laug. Yog li cov condensed qauv qauv ntawm propan-2-ol yog CH3CH(OH)CH3.
Ib yam li ntawd, dab tsi yog condensed structural formula rau ethanol? Ethanol's chemical formula yog C2H6O . Cov tshuaj no tuaj yeem sau ua CH3CH2OH lossis C2H5OH . Nws yog tsim los ntawm cuaj atoms uas suav nrog ob lub carbon (C) atoms, rau hydrogen (H) atoms, thiab ib qho oxygen (O) atom.
Yuav ua li cas koj sau ib tug condensed structural formula rau benzene?
Rau tshuaj benzene , cov molecular formula yog C6H6, thiab yog li ntawd muaj rau atoms ntawm carbon thiab rau atoms ntawm hydrogen nyob rau hauv ib molecule. Ceeb toom tias cov molecular formula yog ib txwm ib co ntau ntawm empirical qauv (6 x CH = C6H6).
Qhov txawv ntawm cov qauv qauv thiab cov qauv condensed yog dab tsi?
1 Teb. Ernest Z. A qauv qauv siv cov kab los qhia cov ntawv cog lus nruab nrab cov atom. A condensed qauv qauv oits feem ntau lossis tag nrho cov ntawv cog lus.
Pom zoo:
Koj sau cov qauv siv Criss Cross li cas?
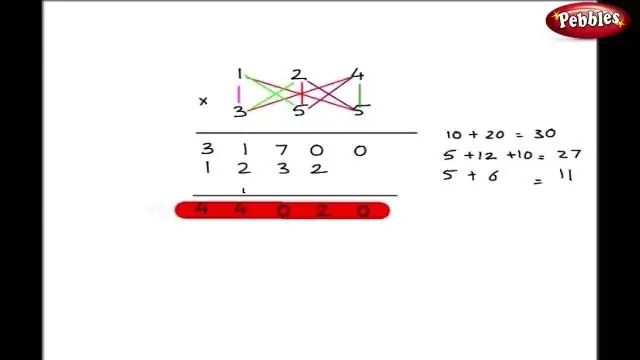
Lwm txoj hauv kev los sau cov qauv kom raug rau ib qho ionic compound yog siv txoj kev crisscross. Hauv txoj kev no, tus lej tus nqi ntawm txhua tus nqi ion raug hla dhau los ua tus lej ntawm lwm ion. Cov cim qhia ntawm cov nqi raug poob. Sau cov qauv rau txhuas (IV) oxide
Tus qauv qauv yog dab tsi Qhov txawv ntawm tus qauv qauv thiab tus qauv molecular yog dab tsi?
Cov mis mos molecular siv cov cim tshuaj thiab cov ntawv sau npe los qhia cov naj npawb ntawm cov atoms sib txawv hauv cov molecule lossis compound. Ib qho empirical formula muab qhov yooj yim tshaj plaws, tag nrho tus lej piv ntawm atoms hauv ib qho chaw. Cov qauv qauv qhia txog kev sib koom ua ke ntawm cov atoms hauv cov molecule
Koj sau cov qauv li cas rau binary ionic compounds?
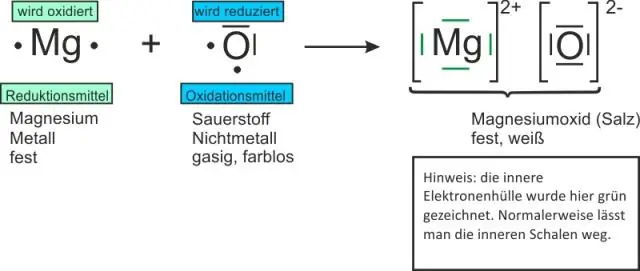
Formulas rau binary compounds pib nrog cov hlau ua raws li cov nonmetal. Cov nqi zoo thiab tsis zoo yuav tsum thim tawm ib leeg. Cov qauv ionic compound yog sau siv qhov qis tshaj ntawm ions
Koj sau cov npe tshuaj thiab cov qauv li cas?
Thaum sau cov mis, qhov zoo atom lossis ion los ua ntej los ntawm lub npe ntawm qhov tsis zoo ion. Lub npe tshuaj rau cov lus ntsev yog sodium chloride. Lub sij hawm lub sij hawm qhia tau hais tias lub cim rau sodium yog Na thiab lub cim rau chlorine yog Cl. Cov tshuaj formula rau sodium chloride yog NaCl
Vim li cas cov qauv hauv daim duab 1 homologous cov qauv?

Lub xub ntiag ntawm homologous qauv qhia tau hais tias cov kab mob hloov zuj zus los ntawm cov poj koob yawm txwv. 1. Xa mus rau daim duab 1. Siv cov ntaub ntawv Table 1, Qhia qhov tseeb ntawm lub cev qhia rau txhua yam kab mob uas tau teev tseg
