
Video: Puas yog Co muaj daim ntawv cog lus covalent?

2024 Tus sau: Miles Stephen | [email protected]. Kawg hloov kho: 2023-12-15 23:36
Carbon monoxide yog ib tug hetero nuclear diatomic molecule. Nws yog a polar covalent molecule raws li electronegativity sib txawv ntawm oxygen thiab carbon yog ntau dua 0.4, li no, daim ntawv a polar covalent bond.
Ua raws li qhov no, hom ntawv cog lus twg yog CO?
Cov pa roj carbon monoxide muaj ib tug carbon atom thiab ib tug oxygen atom, txuas los ntawm ib tug triple daim ntawv cog lus uas muaj ob covalent daim ntawv cog lus as well as ib dative covalent daim ntawv cog lus . Nws yog qhov yooj yim tshaj plaws oxocarbon thiab isoelectronic nrog cov cyanide anion, nitrosonium cation thiab molecular nitrogen.
Ib yam li ntawd, qhov molecular polarity ntawm CO yog dab tsi thiab vim li cas? Oxygen muaj cov electronegativity ntau dua li cov pa roj carbon, yog li nws yuav rub cov electrons hla, ua rau cov pa oxygen ib feem tsis zoo. Tsis zoo li cov pa roj carbon dioxide, tsis muaj oxygen atom thib ob los tiv thaiv kev rub tawm. Yog li ntawd, carbon monoxide yog polar.
Kuj nug, yog co polar lossis nonpolar lossis ionic?
Cov pa roj carbon dioxide ( CO 2) muaj ob polar C = O. bonds, tab sis lub geometry ntawm CO 2 yog linear thiaj li hais tias ob daim ntawv cog lus dipole lub sij hawm tshem tawm thiab tsis muaj net molecular dipole lub sij hawm; molecule yog tsis muaj polar.
Puas yog cov pa roj carbon thiab sulfur yog cov khoom sib txuas?
Qhov C-O daim ntawv cog lus yog considerably polar . Txawm hais tias C thiab S muaj qhov zoo sib xws ntawm electronegativity, S yog me ntsis ntau electronegative tshaj C, thiab yog li C-S daim ntawv cog lus yog me ntsis xwb polar . Vim tias oxygen yog electronegative ntau dua leej faj , oxygen kawg ntawm lub molecule yog qhov tsis zoo kawg.
Pom zoo:
Puas muaj leej twg muaj 4 daim ntawv cog lus?

Sulfur muaj plaub electrons nyob ib ncig ntawm nws nyob rau hauv cov qauv no (ib tug ntawm txhua ntawm nws plaub daim ntawv cog lus) uas istwo electrons tsawg tshaj li tus naj npawb ntawm valence electrons nws yuav muaj ib txwm, thiab xws li nws yog ib tug formal nqi ntawm +2
Puas yog NaCl muaj daim ntawv cog lus uas tsis muaj polar?

Yog lawm, NaCl yog ib daim ntawv cog lus ionic uas ua rau nws polar. Qhov txawv ntawm electronegativities yog dab tsi ua rau daim ntawv cog lus polar lossis nonpolar. Yog hais tias ob lub atoms nyob rau hauv ib daim ntawv cog lus muaj tib lub electronegativity, (xws li, uas muaj ob ntawm tib lub atoms) daim ntawv cog lus yog nonpolar vim tias ob lub atoms muaj qhov sib npaug ntawm cov electrons
Yuav ua li cas yog daim ntawv cog lus sib txawv ntawm daim ntawv cog lus ionic?
Qhov sib txawv ntawm cov ionic thiab covalent daim ntawv cog lus yog tias covalent daim ntawv cog lus yog tsim thaum ob lub atoms sib koom electrons. Ionic bonds yog lub zog uas tuav ua ke electrostatic zog ntawm kev nyiam ntawm cov ions oppositely them. Ionic bonds muaj qhov sib txawv electronegativity ntau dua lossis sib npaug li 2
Dab tsi yog qhov txawv ntawm daim ntawv cog lus lub zog thiab daim ntawv cog lus dissociation zog?
Qhov sib txawv tseem ceeb ntawm kev sib txuas ntawm lub zog thiab lub zog bonddissociation yog tias lub zog sib txuas yog qhov nruab nrab ntawm lub zog xav tau los rhuav tshem tag nrho cov kev sib txuas ntawm tib ob hom atoms nyob rau hauv ib qho chaw sib txuas whereas daim ntawv cog lus dissociation zog yog tus nqi ntawm cov hluav taws xob xav tau los rhuav tshem ib qho kev sib koom ua ke inhomolysis
Puas yog daim ntawv cog lus hydrogen zoo ib yam li daim ntawv cog lus?
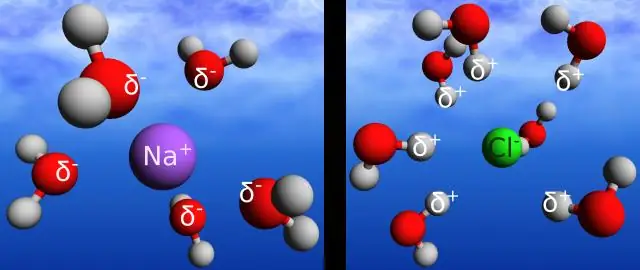
Hydrogen daim ntawv cog lus yog lub npe muab rau electrostatic kev sib cuam tshuam ntawm tus nqi zoo ntawm hydrogen atom thiab tus nqi tsis zoo ntawm oxygen atom ntawm ib qho chaw nyob sib ze. Covalent daim ntawv cog lus yog qhov sib cuam tshuam electrostatic ntawm ob lub atom hauv tib lub molecule
