
Video: Puas yog NaCl muaj daim ntawv cog lus uas tsis muaj polar?

2024 Tus sau: Miles Stephen | [email protected]. Kawg hloov kho: 2023-12-15 23:36
Yog lawm, NaCl yog ib ionic daim ntawv cog lus uas ua rau nws polar. Qhov txawv ntawm electronegativities yog dab tsi ua a daim ntawv cog lus polar lub tsis muaj polar . Yog tias ob lub atom hauv a daim ntawv cog lus muaj tib electronegativity, (piv txwv li, uas muaj ob ntawm tib lub atoms) daim ntawv cog lus yog nonpolar raws li ob qho tib si atom muaj ib tug sib npaug attraction rau electrons.
Kuj nug, NaCl puas muaj daim ntawv cog lus covalent?
Ionic daim ntawv cog lus feem ntau tshwm sim ntawm hlau thiab nonmetal ions. Piv txwv li, sodium (Na), hlau, thiab chloride (Cl), nonmetal, tsim ib qho ionic daim ntawv cog lus ua NaCl . Hauv ib covalent bond , cov atom daim ntawv cog lus los ntawm kev sib koom electrons.
Tom qab ntawd, lo lus nug yog, lub teb chaws twg muaj cov nyiaj tsis muaj polar covalent? Ib qho piv txwv ntawm a nonpolar covalent bond yog cov daim ntawv cog lus ntawm ob lub hydrogen atoms vim tias lawv sib npaug sib faib cov electrons. Lwm qhov piv txwv ntawm a nonpolar covalent bond yog cov daim ntawv cog lus nruab nrab ntawm ob lub chlorine atoms vim lawv kuj sib npaug ntawm cov electrons.
Ua raws li qhov xav tau, vim li cas NaCl tsis yog daim ntawv cog lus?
A sodium chloride ionic daim ntawv cog lus . Qhov zoo them sodium cation thiab qhov tsis zoo chloride anion nyiam tso lawv tus kheej ntawm ib sab vim lawv qhov kev sib nrig sib electrostatic attraction. Vim tsis muaj hluav taws xob sib koom, peb tsis piav txog ib qho ionic daim ntawv cog lus nrog ib txoj kab raws li peb ua rau covalent bonds.
Hom ntawv cog lus twg muaj nyob hauv sodium chloride?
Ib qho ionic compound xws li sodium chloride yog tuav ua ke los ntawm ib qho ionic daim ntawv cog lus . Qhov no hom ntawv cog lus yog tsim thaum oppositely them ions nyiam. Qhov kev nyiam no zoo ib yam li ob tus ncej ntawm cov hlau nplaum. Ib qho ion lossis atom raug them yog tsim thaum lub atom nce lossis poob ib lossis ntau dua electrons.
Pom zoo:
Yuav ua li cas yog daim ntawv cog lus sib txawv ntawm daim ntawv cog lus ionic?
Qhov sib txawv ntawm cov ionic thiab covalent daim ntawv cog lus yog tias covalent daim ntawv cog lus yog tsim thaum ob lub atoms sib koom electrons. Ionic bonds yog lub zog uas tuav ua ke electrostatic zog ntawm kev nyiam ntawm cov ions oppositely them. Ionic bonds muaj qhov sib txawv electronegativity ntau dua lossis sib npaug li 2
Dab tsi yog qhov txawv ntawm daim ntawv cog lus lub zog thiab daim ntawv cog lus dissociation zog?
Qhov sib txawv tseem ceeb ntawm kev sib txuas ntawm lub zog thiab lub zog bonddissociation yog tias lub zog sib txuas yog qhov nruab nrab ntawm lub zog xav tau los rhuav tshem tag nrho cov kev sib txuas ntawm tib ob hom atoms nyob rau hauv ib qho chaw sib txuas whereas daim ntawv cog lus dissociation zog yog tus nqi ntawm cov hluav taws xob xav tau los rhuav tshem ib qho kev sib koom ua ke inhomolysis
Leej twg yog tus qauv ntawm ib qho tsis muaj polar molecule uas muaj cov ntawv cog lus nonpolar?

(1), (3) H2O thiab NH3 yog cov molecules uas muaj polar covalent bonds, tab sis lawv cov khoom siv hluav taws xob tsis sib luag. (4) H2 yog ib tug nonpolar molecule uas muaj ib tug symmetrical tis ntawm electrons, tab sis cov nyiaj ntawm cov hydrogen atoms yog nonpolar covalent
Puas yog daim ntawv cog lus hydrogen zoo ib yam li daim ntawv cog lus?
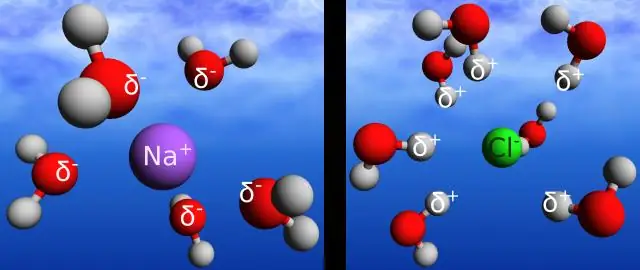
Hydrogen daim ntawv cog lus yog lub npe muab rau electrostatic kev sib cuam tshuam ntawm tus nqi zoo ntawm hydrogen atom thiab tus nqi tsis zoo ntawm oxygen atom ntawm ib qho chaw nyob sib ze. Covalent daim ntawv cog lus yog qhov sib cuam tshuam electrostatic ntawm ob lub atom hauv tib lub molecule
Yuav ua li cas koj txiav txim siab yog tias daim ntawv cog lus yog polar tsis muaj lub rooj electronegativity?

Kauj Ruam 2: Txheeb xyuas txhua daim ntawv cog lus raws li polar lossis nonpolar. (Yog hais tias qhov sib txawv ntawm electronegativity rau cov atoms nyob rau hauv ib daim ntawv cog lus yog ntau tshaj 0.4, peb xav txog daim ntawv cog lus polar. Yog hais tias qhov sib txawv ntawm electronegativity tsawg dua 0.4, daim ntawv cog lus yog qhov tseem ceeb nonpolar.) Yog tias tsis muaj polar bonds, lub molecule yog tsis muaj polar
