
Video: Dab tsi yog electron configuration rau calcium atom?

2024 Tus sau: Miles Stephen | [email protected]. Kawg hloov kho: 2023-12-15 23:36
[A] 4s²
Hauv qhov no, qhov kev teeb tsa hluav taws xob ntawm qhov nruab nrab calcium atom yog dab tsi?
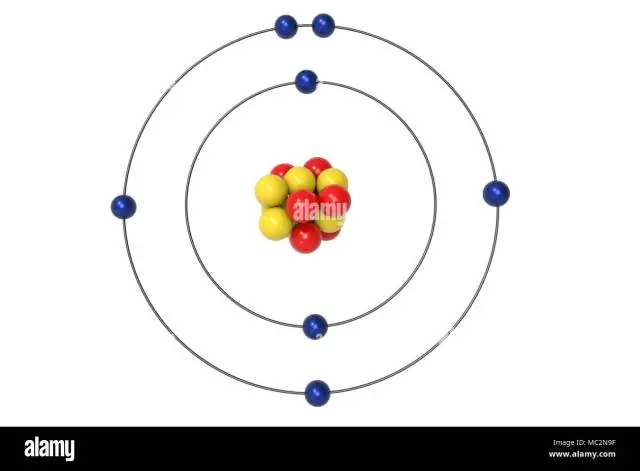
Cov atomic tus lej ntawm calcium yog 20. Qhov no txhais tau tias nyob rau hauv a nruab nrab calcium atom , muaj 20 protons hauv nws cov nucleus. A nruab nrab calcium atom tseem muaj 20 electrons . Cov electron configuration ntawm ib tug nruab nrab calcium atom yog 1s22s22p63s23p64s2.
Calcium atom yog dab tsi? Calcium yog ib yam tshuaj nyob rau hauv lub sij hawm uas muaj lub cim Ca thiab atomic num 20. Calcium yog ib tug mos grey alkaline ntiaj teb hlau uas yog siv los ua ib tug txo tus neeg sawv cev nyob rau hauv lub extraction ntawm thorium, zirconium thiab uranium. Lub caij no kuj yog lub thib tsib tshaj plaws nyob rau hauv lub ntiaj teb crust.
Hais txog qhov no, qhov kev teeb tsa hluav taws xob rau calcium atomic tooj 20 yog dab tsi?
Piv txwv li, hauv av xeev electronic configuration ntawm calcium (Z = 20 ) yog 1s 22s ib 22 ib p 63s ib 23 ua p 64s ib 2. Cov calcium ion ( Ca 2+), txawm li cas los xij, muaj ob electrons tsawg.
Lub caij twg muaj qhov zoo tshaj plaws electronegativity?
Electronegativity nce hauv qab mus rau sab saum toj hauv pab pawg, thiab nce los ntawm sab laug mus rau sab xis hla lub sijhawm. Yog li, tshuaj fluorine yog lub feem ntau electronegative keeb, thaum francium yog ib qho ntawm qhov tsawg tshaj plaws electronegative.
Pom zoo:
Dab tsi yog electron configuration ntawm chlorine nyob rau hauv ib tug zoo siab lub xeev?

Qhov twg electron configuration sawv cev rau ib tug atom ntawm chlorine nyob rau hauv ib tug zoo siab lub xeev? (2) 2-8-6-1 qhov no yog lub xeev zoo siab ntawm Chlorine, nyob rau hauv lub sij hawm lub sij hawm lub xeev av yog 2-8-7. Lub xeev zoo siab electron configuration yog qhia ib tug electron tawm hauv ib theem zog thiab nce mus rau ib theem siab dua
Dab tsi yog qhov tseem ceeb valence electron configuration rau nitrogen?
Qhov seem peb electrons yuav mus rau hauv 2p orbital. Yog li N electron configuration yuav yog 1s22s22p3. Daim ntawv teev npe rau Nitrogen (N) muab txoj hauv kev yooj yim rau cov kws tshawb fawb los sau thiab sib txuas lus li cas electrons tau teeb tsa nyob ib puag ncig lub nucleus ntawm Nitrogen atom
Dab tsi yog qhov ua tiav hauv lub xeev electron configuration rau lub gallium atom?

Hauv av xeev electron configuration ntawm hauv av xeev gaseous nruab nrab gallium yog [Ar]. 3d10 ua. 4s2 ua. 4p1 thiab lub sij hawm cim yog 2P1/2
Cov tshuaj tiv thaiv dab tsi yog calcium carbonate calcium oxide carbon dioxide?

Calcium carbonate tau kub siab kom txog thaum nws dhau los ua thermal decomposition los tsim calcium oxide thiab carbon dioxide. Cov calcium oxide (unslaked txiv qaub) yog yaj hauv dej los tsim calcium hydroxide (limewater). Bubbling carbon dioxide los ntawm qhov no ua ib qho kev ncua ntawm calcium carbonate
Qhov twg electron configuration sawv cev rau ib tug atom nyob rau hauv nws lub hauv av?

Yog li txhua qhov kev teeb tsa hluav taws xob nyob rau hauv uas lub xeem electron (dua, lub valence electron) yog nyob rau hauv lub zog ntau dua orbital, lub caij no tau hais tias nyob rau hauv lub xeev zoo siab. Piv txwv li, yog tias peb saib hauv av hauv lub xeev (electrons nyob rau hauv lub zog qis tshaj plaws uas muaj orbital) ntawm oxygen, lub teeb tsa hluav taws xob yog 1s22s22p4
