Cov txheej txheem:
Video: Dab tsi yog empirical formula thiab molecular mis?

2024 Tus sau: Miles Stephen | [email protected]. Kawg hloov kho: 2023-12-15 23:36
Cov qauv molecular qhia rau koj paub pes tsawg atoms ntawm txhua lub ntsiab lus nyob rau hauv ib lub compound, thiab cov qauv empirical Qhia rau koj paub qhov yooj yim tshaj plaws los yog feem ntau txo qhov piv ntawm cov ntsiab lus nyob rau hauv ib tug compound. Yog ib tug compound molecular formula tsis tuaj yeem txo qis dua, ces tus empirical formula yog tib yam li cov molecular formula.
Tsuas yog li ntawd, koj yuav ua li cas thiaj nrhiav tau cov mis molecular los ntawm cov mis empirical?
Faib cov molar loj ntawm lub compound los ntawm lub empirical formula pawg. Cov txiaj ntsig yuav tsum yog tus lej tag nrho lossis ze rau tag nrho tus lej. Muab tag nrho cov subscripts nyob rau hauv lub empirical formula los ntawm tag nrho cov naj npawb pom nyob rau hauv kauj ruam 2. Qhov tshwm sim yog qhov molecular formula.
Dab tsi yog cov qauv qhia txog kev sib xyaw uas nws cov qauv molecular yog s6o9? yog tias Cov mis mos molecular yog S6O9 kom tau empirical formula peb nrhiav tus lej uas yuav faib ua 6 thiab 9 mus rau tsib qhov tsawg tshaj plaws tag nrho tus lej piv (uas yog lub ntsiab lus ntawm empirical formula !).
Tsuas yog li ntawd, koj yuav ua li cas thiaj nrhiav tau cov mis molecular los ntawm cov mis empirical thiab molar mass?
Empirical formula hnyav = (1 x 12.01g/mol) + (2 x 1.01g/mol) + (1 x 16.00g/mol) = 30.02g/mol. Faib cov molar loj rau cov molecular formula los ntawm empirical formula mass . Qhov tshwm sim txiav txim siab pes tsawg zaus los muab cov ntawv sau rau hauv empirical formula kom tau molecular formula.
Yuav ua li cas koj daws rau empirical formula?
Kev suav ntawm ib qho Empirical Formula
- Kauj Ruam 1: Tau qhov loj ntawm txhua lub ntsiab lus hauv grams. Element % = mass in g = m.
- Kauj Ruam 2: Txiav txim seb tus naj npawb ntawm moles ntawm txhua hom atom tam sim no.
- Kauj Ruam 3: Faib tus naj npawb ntawm moles ntawm txhua lub caij los ntawm tus lej tsawg tshaj plaws ntawm moles.
- Kauj Ruam 4: Hloov cov lej rau tag nrho cov lej.
Pom zoo:
Dab tsi yog cov khoom lag luam hauv kev sib npaug molecular rau kev ua tiav cov tshuaj tiv thaiv ntawm aqueous barium hydroxide thiab nitric acid?

Ba(OH)2 + 2HNO3 → Ba(NO3)2 + 2H2O. Barium hydroxide react nrog nitric acid los tsim barium nitrate thiab dej
Dab tsi yog cov mis empirical rau caffeine?
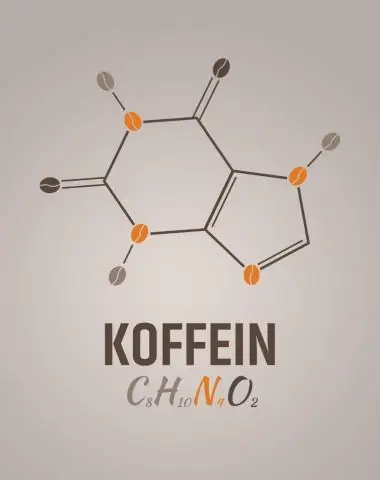
2 Teb. C8H10N4O2 yog cov mis molecular forcaffeine
Dab tsi yog qhov txawv ntawm molecular solids thiab covalent solids?

Molecular solids - Ua los ntawm atoms ormolecules tuav ua ke los ntawm London dispersion rog, dipole-dipoleforces, los yog hydrogen bonds. Ib qho piv txwv ntawm molecular solidis sucrose. Covalent-network (tseem hu ua atomic) cov khoom-ua los ntawm cov atoms txuas nrog covalentbonds; cov intermolecular rog yog covalent bonds thiab
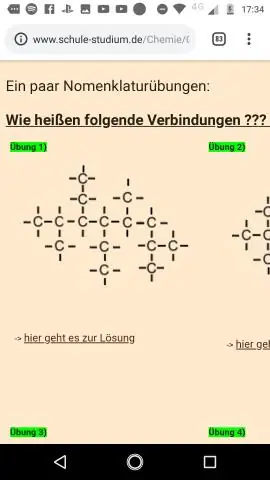
Tus qauv qauv yog dab tsi Qhov txawv ntawm tus qauv qauv thiab tus qauv molecular yog dab tsi?
Cov mis mos molecular siv cov cim tshuaj thiab cov ntawv sau npe los qhia cov naj npawb ntawm cov atoms sib txawv hauv cov molecule lossis compound. Ib qho empirical formula muab qhov yooj yim tshaj plaws, tag nrho tus lej piv ntawm atoms hauv ib qho chaw. Cov qauv qauv qhia txog kev sib koom ua ke ntawm cov atoms hauv cov molecule
Dab tsi yog empirical formula ntawm ib qho compound?

Cov mis empirical ntawm ib qho compound yog qhov yooj yim tshaj plaws tus lej piv ntawm txhua hom atom hauv ib qho chaw. Nws tuaj yeem yog tib yam li cov qauv molecular, tab sis tsis tas li. Cov mis empirical tuaj yeem suav los ntawm cov ntaub ntawv hais txog qhov loj ntawm txhua lub ntsiab lus hauv ib qho chaw lossis los ntawm qhov feem pua
