Cov txheej txheem:

Video: Lub cev molecular ntawm cov molecule hauv qab no yog dab tsi?

2024 Tus sau: Miles Stephen | [email protected]. Kawg hloov kho: 2023-12-15 23:36
Yog hais tias tag nrho cov no yog cov khub niam txiv molecular geometry yog tetrahedral (piv txwv li CH4). Yog hais tias muaj ib leeg ib khub ntawm electrons thiab peb daim ntawv cog lus khub qhov tshwm sim molecular geometry yog trigonal pyramidal (piv txwv li NH3). Yog hais tias muaj ob daim ntawv cog lus thiab ob leeg ib leeg ntawm electrons molecular geometry yog angular lossis bent (piv txwv li H2O).
Ua kom pom qhov no, cov duab ntawm cov molecules yog dab tsi?
Lub tsib zoo tagnrho cov duab yog: linear, trigonal planar, tetrahedral, trigonal bypramidal thiab octahedral. Ib qho tseem ceeb uas yuav tsum nco ntsoov txog molecular zoo yog tias tag nrho cov diatomic (sib xyaw nrog ob lub atoms) cov kab sib txuas. Yog li H2, HCl thiab Cl2 yog tag nrho cov kab.
Dab tsi yuav ua rau cov duab ntawm lub molecule ua tetrahedral? A molecule yog tetrahedral yog lub hauv paus atom muaj plaub daim ntawv cog lus thiab tsis muaj ib khub. Kev piav qhia: Ib qho piv txwv yog a molecule ntawm methane (saib daim duab). Cov electron khub hauv daim ntawv cog lus tawm tsam cov electrons hauv lwm daim ntawv cog lus, yog li lawv txhua tus sim kom deb ntawm ib leeg li sai tau.
Yog li ntawd, koj yuav ua li cas thiaj nrhiav tau cov duab molecular?
Cov kauj ruam siv los nrhiav cov duab ntawm cov molecule
- Kos tus qauv Lewis.
- Suav tus naj npawb ntawm cov pab pawg electron thiab txheeb xyuas lawv raws li daim ntawv cog lus ntawm pawg hluav taws xob lossis ib leeg ntawm cov hluav taws xob.
- Lub npe electron-group geometry.
- Saib ntawm txoj hauj lwm ntawm lwm cov atomic nuclei nyob ib ncig ntawm lub hauv paus txiav txim siab cov molecular geometry.
Dab tsi yog 5 cov duab yooj yim ntawm cov molecules?
Molecular Geometry. VSEPR txoj kev xav piav qhia tsib lub ntsiab ntawm cov qauv yooj yim: linear, trigonal planar, tetrahedral , trigonal bipyramidal, thiab octahedral.
Pom zoo:
Lub luag haujlwm ntawm CDK yog dab tsi hauv lub cev ua haujlwm ntawm tes tshwj xeeb hauv lub voj voog ntawm tes?

Los ntawm phosphorylation, Cdks teeb liab lub xov tooj ntawm tes uas nws tau npaj kom dhau mus rau theem tom ntej ntawm lub voj voog ntawm tes. Raws li lawv lub npe qhia, Cyclin-Dependent Protein Kinases yog nyob ntawm cyclins, lwm chav kawm ntawm kev tswj hwm cov protein. Cyclins khi rau Cdks, ua kom cov Cdks rau phosphorylate lwm cov molecules
Lub npe ntawm lub network loj hauv lub cev yog dab tsi uas tswj cov noob qhia?

NARRATOR: Cov cim npe no thiab lwm tus tswj cov noob qhia los ntawm lub network loj hauv lub cev hu ua epigenome. RANDY JIRTLE: Epigenetics txhais tau tias tsuas yog lub ntsiab lus saum genome
Dab tsi yog qhov tseeb ntawm lub cev lub cev lub cev kev xav xav?

Lub cev lub cev lub ntsiab lus kev xav (PSSH) yog ib txoj hauj lwm hauv lub tswv yim ntawm kev txawj ntse tsim los ntawm Allen Newell thiab Herbert A. 'Ib lub cim lub cev muaj qhov tsim nyog thiab txaus txhais tau tias rau kev ua haujlwm ntse.'
Dab tsi ntawm cov hauv qab no muaj nyob rau hauv cov tsiaj hlwb tab sis tsis yog cov nroj tsuag?
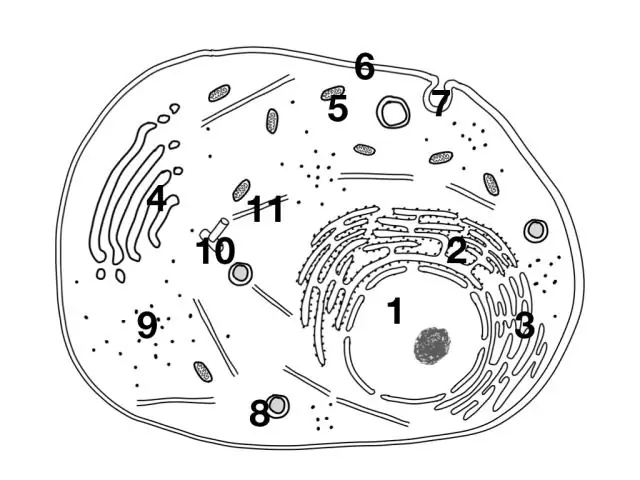
Mitochondria, Cell Phab ntsa, Cell membrane, Chloroplast, Cytoplasm, Vacuole. Lub cell phab ntsa, chloroplasts thiab vacuole muaj nyob rau hauv cov nroj tsuag cell es tsis yog tsiaj hlwb
Lub luag haujlwm ntawm lub cev thiab lub cev yog dab tsi?

Anatomy yog kev kawm txog cov qauv thiab kev sib raug zoo ntawm lub cev. Physiology yog kev kawm txog kev ua haujlwm ntawm lub cev thiab lub cev tag nrho
