Cov txheej txheem:

Video: Koj npe binary covalent compounds li cas?

2024 Tus sau: Miles Stephen | [email protected]. Kawg hloov kho: 2024-01-18 08:15
Sau npe Binary Covalent Compounds
- Lub npe cov non-metal furthest mus rau sab laug ntawm lub periodic rooj los ntawm nws cov elemental npe .
- Lub npe lwm yam uas tsis yog-hlau los ntawm nws cov elemental npe thiab ib-ide xaus.
- Siv cov prefixes mono-, di-, tri-. los qhia tus naj npawb ntawm cov khoom ntawd hauv cov molecule.
- Yog tias mono yog thawj lub npe, nws nkag siab thiab tsis sau.
Tsis tas li ntawd, ua li cas koj lub npe binary compounds?
Qhov kev txiav txim rau npe hauv a binary compound yog thawj tus cation, ces anion. Siv cov npe ntawm cation nrog ib tug ruaj oxidation xeev ncaj qha los ntawm lub periodic rooj. Cov npe ntawm cov anion yuav ua los ntawm cov hauv paus hniav ntawm lub caij npe ntxiv rau cov lus "-ide."
Ib yam li ntawd, NaCl puas yog binary compound? Hauv chemistry, a binary compound yog ib yam dab tsi uas muaj ob lub ntsiab. Hauv ib binary compound , tej zaum tsuas muaj ib qho ntawm txhua lub ntsiab lus. Peb pom qhov no nrog sodium chloride (nqaij) NaCl , uas muaj ib qho sodium (Na) thiab ib qho tshuaj chlorine (Cl).
Tsis tas li ntawd kom paub yog, thaum naming binary covalent compound uas lub npe hu ua thawj?
Lub npe binary (ob- keeb ) covalent compounds zoo ib yam li npe yooj yim ionic tshuaj sib xyaw . Cov thawj lub ntsiab nyob rau hauv cov mis tsuas yog teev siv lub npe ntawm tus keeb . Qhov thib ob element npe los ntawm kev noj cov qia ntawm lub keeb lub npe thiab ntxiv lub ntsiab lus -ide.
Hom 3 compounds yog dab tsi?
Hom III binary tshuaj sib xyaw tsis muaj hlau atoms. Muaj ob lub npe sib txawv rau Hom III binary tshuaj sib xyaw : "laus system" thiab "new system." Cov txheej txheem qub siv cov ntawv ua ntej los qhia tus lej ntawm txhua lub atom tam sim no thiab cov txheej txheem tshiab zoo ib yam rau cov uas siv rau npe. Hom II tshuaj sib xyaw.
Pom zoo:
Yuav ua li cas koj lub npe compounds nyob rau hauv Khan Academy?

Rau binary ionic compounds (ionic compounds uas tsuas muaj ob hom ntawm cov ntsiab lus), cov tebchaw muaj npe los ntawm kev sau lub npe ntawm cov cation ua ntej tom qab lub npe ntawm cov anion
Dab tsi yog ob hom loj ntawm binary chemical compounds?

Ib qho binaiy compound tsuas muaj ob lub ntsiab lus. Cov hom loj ntawm binary compounds yog ionic (cov khoom sib xyaw uas muaj cov hlau thiab cov hlau tsis muaj hlau) thiab nonionic (cov khoom uas muaj ob lub nonmetals)
Organic compounds thiab inorganic compounds yog dab tsi?

Lub ntsiab sib txawv yog nyob rau hauv lub xub ntiag ntawm ib tug carbon atom; cov organic tebchaw yuav muaj cov pa roj carbon atom (thiab feem ntau yog hydrogen atom, tsim hydrocarbons), thaum yuav luag tag nrho cov inorganic tebchaw tsis muaj ib qho ntawm ob lub atoms. Meanwhile, inorganic tebchaw muaj xws li cov ntsev, hlau, thiab lwm yam elemental compounds
Koj npe ionic compounds piv txwv li cas?

Rau binary ionic compounds (ionic compounds uas muaj tsuas yog ob hom ntawm cov ntsiab lus), cov tebchaw muaj npe los ntawm kev sau lub npe ntawm cov cation ua ntej tom qab lub npe ntawm anion. Piv txwv li, KCl, ib qho ionic compound uas muaj K + thiab Cl-ions, yog lub npe hu ua potassium chloride
Koj sau cov qauv li cas rau binary ionic compounds?
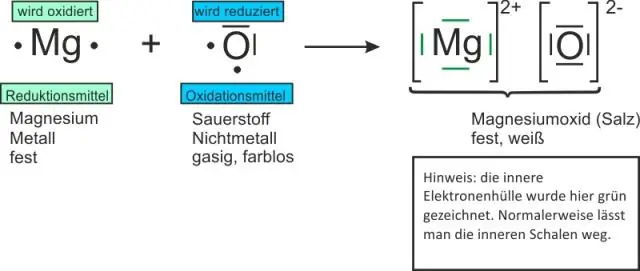
Formulas rau binary compounds pib nrog cov hlau ua raws li cov nonmetal. Cov nqi zoo thiab tsis zoo yuav tsum thim tawm ib leeg. Cov qauv ionic compound yog sau siv qhov qis tshaj ntawm ions
