
Video: Electron configuration ntawm s2 - yog dab tsi?

2024 Tus sau: Miles Stephen | [email protected]. Kawg hloov kho: 2023-12-15 23:36
Cov S2 - ion, qhov yooj yim sulfur anion thiab tseem hu ua sulfide, muaj ib qho electron configuration ntawm 1s2 2s2 2p6 3s2 3p6. Ib qho nruab nrab atom ntawm sulfur muaj 16 electrons , tab sis lub atom ces nce ib qho ntxiv ob electrons thaum nws tsim ib ion, noj tag nrho cov naj npawb ntawm electrons rau 18.
Ib yam li ntawd, tib neeg nug, dab tsi yog lub hauv paus xeev electron teeb tsa ntawm ion s2 −?
S: 1s2 2s2 2p6 3s2 3p4 S2 -: 1s2 2s2 2p6 3s2 3p6 Nco tseg: Hloov 1s2 2s2 2p6 los ntawm [Ne] tau txais. Ib qho point yog khwv tau rau qhov tseeb kev teeb tsa rau S.
Tsis tas li ntawd, lub npe tiag tiag rau s2 ion yog dab tsi? Sulfide (Lus Askiv Askiv kuj sulfide) yog ib qho inorganic anion ntawm sulfur nrog cov tshuaj formula S2− los yog ib tug compound uas muaj ib los yog ntau tshaj S2− ions.
Kuj nug, pes tsawg electrons nyob hauv ion s2 -?
18 electrons
Qhov twg loj dua Ca lossis ca2+?
Ca atom yuav loj dua tshaj Ca2+ vim cations me me tshaj lawv niam txiv atom. Cov Ca2+ cation yog loj dua tshaj qhov Mg2+ cation txij thaum lub calcium cation muaj ib theem ua tiav ntau dua li magnesium cation.
Pom zoo:
Dab tsi ntawm magnification yuav ua tiav los ntawm lub teeb vs electron microscopes?
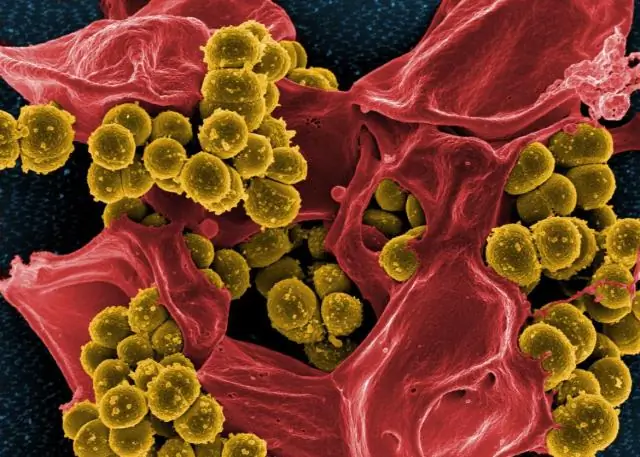
Lub tshuab xa hluav taws xob xaim hluav taws xob tau ua tiav zoo dua 50 teev kev daws teeb meem hauv annular dark-field imaging hom thiab magnifications txog li 10,000,000 × whereas feem ntau lub teeb microscopes raug txwv los ntawm diffraction txog li 200 nm daws teeb meem thiab muaj txiaj ntsig zoo hauv qab 2000 ×
Yuav ua li cas electron configuration muaj feem xyuam rau quantum tooj?
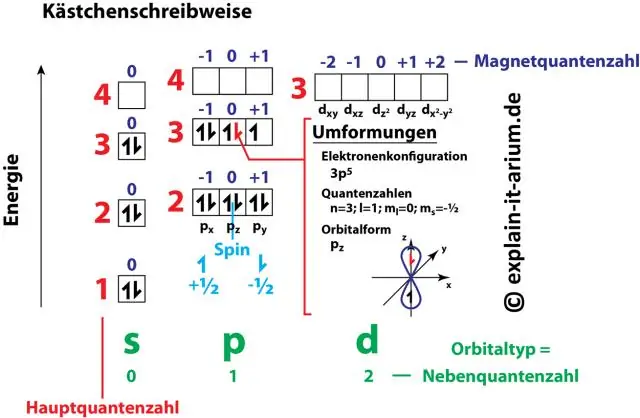
Tus lej thiab tsab ntawv ua khub hauv ib qho kev teeb tsa hluav taws xob sawv cev rau ob ntawm cov hluav taws xob plaub tus lej quantum. Cov lej quantum no qhia peb cov ntaub ntawv ntau ntxiv txog cov khoom ntawm electrons thiab lawv cov orbitals. Tus thawj quantum tooj (n) qhia peb ib tug electron lub zog theem thiab nws loj
Yuav ua li cas koj nrhiav tau lub electron configuration rau oxygen?

Hauv kev sau cov khoom siv hluav taws xob rau cov pa oxygen thawj ob lub electrons yuav mus rau hauv 1s orbital. Txij li thaum 1s tsuas tuav tau ob lub electrons tom ntej no 2 electrons rau O mus rau hauv 2s orbital. Cov plaub electrons ntxiv yuav mus rau hauv 2p orbital. Yog li O electron configuration yuav yog 1s22s22p4
Yuav ua li cas koj nrhiav tau tus electron configuration rau nyiaj?

Hauv av xeev electron configuration ntawm hauv av xeev gaseous nruab nrab nyiaj yog [Kr]. 4d10 ua. 5s1 thiab lub sij hawm cim yog 2S1/2
Qhov twg electron configuration sawv cev rau ib tug atom nyob rau hauv nws lub hauv av?

Yog li txhua qhov kev teeb tsa hluav taws xob nyob rau hauv uas lub xeem electron (dua, lub valence electron) yog nyob rau hauv lub zog ntau dua orbital, lub caij no tau hais tias nyob rau hauv lub xeev zoo siab. Piv txwv li, yog tias peb saib hauv av hauv lub xeev (electrons nyob rau hauv lub zog qis tshaj plaws uas muaj orbital) ntawm oxygen, lub teeb tsa hluav taws xob yog 1s22s22p4
