
Video: Qhov nruab nrab twg yog tsim los ntawm br2 rau alkene?

2024 Tus sau: Miles Stephen | [email protected]. Kawg hloov kho: 2023-12-15 23:36
Description: Kev kho mob ntawm alkenes nrog bromine ( Br2 ) muab vicinal dibromides (1, 2-dibromides). Lus Cim: Cov bromines ntxiv rau lub ntsej muag ntawm ob daim ntawv cog lus ( anti ntxiv ”). Qee lub sij hawm cov kuab tshuaj tau hais hauv cov tshuaj tiv thaiv no - ib qho hnyav hnyav yog carbon tetrachloride (CCl4).
Yog li ntawd, dab tsi intermediates koom nrog hauv bromination ntawm alkene?
Kev tshwm sim Txheej txheem cej luam : Cov tshuaj tiv thaiv alkene halogenation, tshwj xeeb tshaj yog bromination lossis chlorination, yog ib qho uas dihalide xws li Cl2 lossis Br2 tau ntxiv rau cov molecule tom qab rhuav tshem cov pa roj carbon monoxide ob daim ntawv cog lus. Cov halides ntxiv rau cov carbons nyob sib ze los ntawm lub ntsej muag ntawm cov molecule.
Ib yam li ntawd, uas alkene yuav muab meso daim ntawv nrog br2? 1 Teb. Ernest Z. trans-Hex-3-ene daim ntawv a meso sib thaum nws reacts nrog bromine . Lub bromination ntawm ib alkene Kev koom tes nrog kev tsim ntawm ib qho nruab nrab cyclic bromonium ion, ua raws li kev tiv thaiv ntxiv ntawm ib qho bromide ion ua daim ntawv cov khoom.
Tsis tas li ntawd, yuav ua li cas thaum br2 reacts nrog alkene?
Alkenes teb hauv qhov txias nrog cov kua ntshiab bromine , los yog nrog ib tug daws ntawm bromine nyob rau hauv cov organic hnyav xws li tetrachloromethane. Ob daim ntawv cog lus tawg, thiab a bromine atom ua txuas rau txhua cov pa roj carbon. Cov bromine poob nws cov xim liab-xim av kom muab cov kua tsis muaj xim.
Dab tsi yog thawj qhov nruab nrab hauv bromination ntawm alkene?
Cov tshuaj tiv thaiv mechanism rau ib alkene bromination tuaj yeem piav raws li hauv qab no. Hauv ua ntej kauj ruam ntawm cov tshuaj tiv thaiv, ib tug bromine molecule mus rau electron nplua nuj alkene carbon-carbon ob daim ntawv cog lus.
Pom zoo:
Yuav ua li cas koj pom qhov nruab nrab thiab nruab nrab ntawm daim duab peb sab?

Txhawm rau nrhiav qhov chaw nruab nrab ntawm daim duab peb sab, nws yog qhov yooj yim tshaj plaws los kos tag nrho peb qhov nruab nrab thiab nrhiav lawv qhov kev sib tshuam. Txhawm rau kos qhov nruab nrab ntawm daim duab peb sab, ua ntej nrhiav qhov nruab nrab ntawm ib sab ntawm daim duab peb sab. Kos ib kab ntu uas txuas qhov taw tes no mus rau qhov rov qab vertex
Qhov sib npaug sib npaug rau qhov nruab nrab ntawm h2so4 los ntawm Koh yog dab tsi?

Hauv daim vis dis aus no peb yuav sib npaug ntawm qhov sib npaug KOH + H2SO4 = K2SO4 + H2O thiab muab cov coefficients raug rau txhua qhov sib xyaw. Txhawm rau sib npaug KOH + H2SO4 = K2SO4 + H2O koj yuav tsum nco ntsoov suav tag nrho cov atoms ntawm txhua sab ntawm cov tshuaj sib npaug
Qhov nruab nrab nruab nrab ntawm lub ntiaj teb yog dab tsi?
Lub teb chaws qhov ntsuas kub yog 2.91 ° C (5.24 ° F) saum toj no 1961-1990 qhov nruab nrab, tawg cov ntaub ntawv yav dhau los tau teev tseg hauv 2013 los ntawm 0.99 ° C (1.78 ° F)
Qhov kev sib npaug twg yog qhov tsim nyog los suav cov cua sov uas tsim los ntawm HCl NaOH cov tshuaj tiv thaiv?
Xam cov naj npawb ntawm moles ntawm lub hauv paus koj ntxiv los txiav txim siab molar tshav kub ntawm qhov nruab nrab, qhia siv qhov sib npaug Δ H = Q ÷ n, qhov twg 'n' yog tus naj npawb ntawm moles. Piv txwv li, xav tias koj ntxiv 25 mL ntawm 1.0 M NaOH rau koj HCl los ua kom muaj cua sov ntawm nruab nrab ntawm 447.78 Joules
Koj pom qhov nruab nrab thiab nruab nrab hauv tableau li cas?
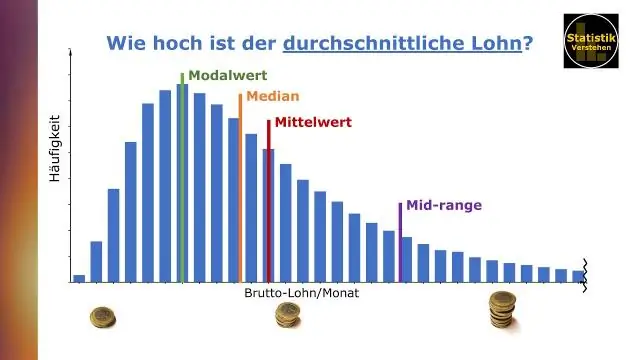
Kev sib txuas lus cov ntaub ntawv nrog Tableau los ntawm Ben Jones Qhov nruab nrab (lossis nruab nrab) yog txiav txim siab los ntawm kev suav tag nrho cov txiaj ntsig hauv cov ntaub ntawv teev thiab faib los ntawm tus lej ntawm qhov tseem ceeb. Qhov nruab nrab yog tus nqi nruab nrab hauv cov ntaub ntawv teev nyob rau hauv uas qhov tseem ceeb tau muab tso rau hauv kev txiav txim ntawm qhov loj
