
Video: Qhov sib npaug sib npaug rau qhov nruab nrab ntawm h2so4 los ntawm Koh yog dab tsi?

2024 Tus sau: Miles Stephen | [email protected]. Kawg hloov kho: 2023-12-15 23:36
Hauv daim vis dis aus no peb yuav sib npaug qhov sib npaug KOH + H2SO4 = K2SO 4 + H2O thiab muab cov coefficients raug rau txhua qhov sib xyaw. KOH + H2SO4 = K2SO 4 + H2O Koj yuav tsum nco ntsoov suav tag nrho cov atoms ntawm txhua sab tshuaj sib npaug.
Xav txog qhov no, qhov sib npaug sib npaug rau h2so4 thiab Koh yog dab tsi?
Chemical Equation Balancer H2SO4 + KOH = K2SO4 + H2O.
Kuj Paub, qhov sib npaug sib npaug rau Koh h3po4 yog dab tsi? Tshuaj Kev sib npaug Balancer KOH + H3PO 4 = K3PO4 + H2O.
Ua raws li qhov no hauv kev txiav txim siab, dab tsi yog cov khoom ntawm cov tshuaj tiv thaiv nruab nrab ntawm h2so4 thiab Koh?
Neutralization cov tshuaj tiv thaiv . Ob moles ntawm poov tshuaj hydroxide , lub hauv paus muaj zog, ntawm tshuaj tiv thaiv nrog ib tug mole ntawm muaj zog acid, sulfuric acid , tsim poov tshuaj sulfate thiab dej. Ib mole KOH reacts nrog ib nrab mole ntawm H2SO4 H 2 S O 4.
Yuav ua li cas thaum sulfuric acid reacts nrog poov tshuaj hydroxide?
Potassium hydroxide reacts nrog sulfuric acid Hauv cov txheej txheem uas tuaj yeem piav qhia los ntawm qhov sib npaug: 2KOH + H2SO4 K2SO4 + 2 H2O Yog tias 425 mL ntawm 0.440M H2SO4 reacts nrog 450 mL ntawm 0.210M KOH , dab tsi yog qhov concentration ntawm H2SO4 tshuav tom qab lub tshuaj tiv thaiv ua tiav?
Pom zoo:
Yuav daws qhov tsis sib npaug ntawm cov kab sib npaug thiab cov kab sib npaug sib npaug li cas?

Kev daws qhov tsis sib npaug ntawm qhov sib npaug yog zoo ib yam li kev daws cov kab sib npaug. Qhov sib txawv tseem ceeb yog koj tig lub cim tsis sib xws thaum faib lossis muab faib los ntawm tus lej tsis zoo. Graphing linear inequalities muaj ob peb qhov sib txawv ntxiv. Qhov uas yog qhov ntxoov ntxoo muaj xws li qhov tseem ceeb uas qhov kev tsis sib xws ntawm cov kab tawm muaj tseeb
Qhov nruab nrab nruab nrab ntawm lub ntiaj teb yog dab tsi?
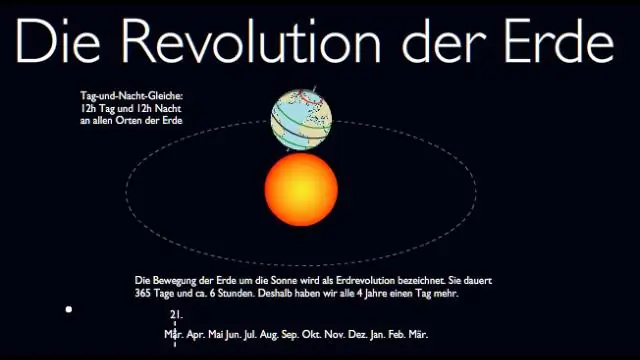
Lub teb chaws qhov ntsuas kub yog 2.91 ° C (5.24 ° F) saum toj no 1961-1990 qhov nruab nrab, tawg cov ntaub ntawv yav dhau los tau teev tseg hauv 2013 los ntawm 0.99 ° C (1.78 ° F)
Dab tsi yog qhov txawv ntawm instantaneous thiab nruab nrab ceev dab tsi yog qhov piv txwv loj tshaj ntawm kev ceev ceev?
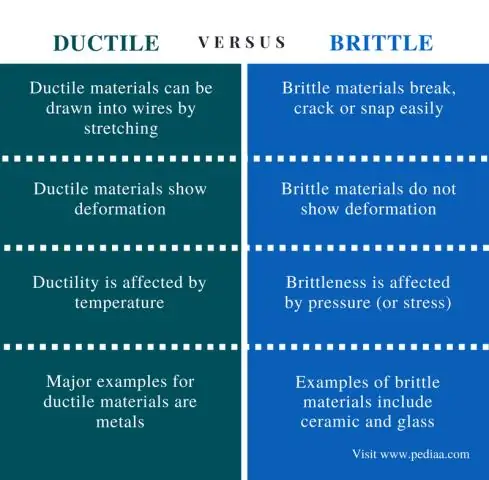
Qhov nruab nrab ceev yog qhov ceev nruab nrab ntawm lub sijhawm. Instantaneous ceev yuav yog qhov ceev txhua qhov muab tam sim ntawd hauv lub sijhawm ntawd, ntsuas nrog lub ntsuas ntsuas lub sijhawm
Dab tsi yog qhov sib txawv ntawm cov kab lus sib npaug thiab qhov sib npaug sib npaug?
Cov kab lus sib npaug muaj qhov muaj txiaj ntsig zoo ib yam tab sis tau nthuav tawm nyob rau hauv ib hom ntawv sib txawv uas siv cov khoom ntawm cov lej xws li ax + bx = (a + b) x yog cov kab lus sib npaug. nruj me ntsis, lawv tsis yog 'sib npaug', yog li peb yuav tsum siv 3 kab sib luag hauv 'sib npaug' es tsis yog 2 raws li qhia ntawm no
Koj ua li cas sib npaug redox cov tshuaj tiv thaiv hauv acidic thiab nruab nrab nruab nrab?

Acid Conditions Solution. Kauj Ruam 1: Sib cais ib nrab cov lus teb. Kauj Ruam 2: Ntsuas cov ntsiab lus uas tsis yog O thiab H. Kauj Ruam 3: Ntxiv H2O kom sib npaug ntawm cov pa. Kauj Ruam 4: Sib npaug hydrogen los ntawm kev ntxiv protons (H +). Kauj Ruam 5: Ntsuas tus nqi ntawm txhua qhov sib npaug nrog electrons. Kauj Ruam 6: Ntsuas cov tshuaj tiv thaiv kom cov electrons sib npaug
