
Video: Base ntsev yog dab tsi?

2024 Tus sau: Miles Stephen | [email protected]. Kawg hloov kho: 2023-12-15 23:36
PIB Electrolyte Ntsev muab cov neeg ncaws pob nrog cov khoom siv electrolyte zoo dua, ua rau lawv ua kom muaj kev ua tau zoo tshaj plaws, nyob twj ywm hydrated thiab tawm tsam qaug zog. BASE ntsev Daim ntawv crystalline tshwj xeeb yog yooj yim digested, sai absorbed thiab muaj nyob rau hauv cov hlab ntsha kom ntxiv cov electrolytes poob hauv hws.
Tsis tas li ntawd, lub rooj ntsev yog cov kua qaub lossis lub hauv paus?
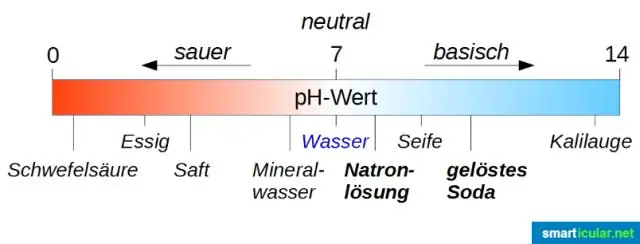
Rooj ntsev yog cov khoom tsim los ntawm neutralization ntawm ib qho kua qaub los ntawm a puag . Yog li nws tsis yog Acid tsis yog Puag . Koj tuaj yeem siv pH Scale los qhia seb nws kua qaub los yog puag.
sodium ntau npaum li cas hauv cov tshuaj ntsev? Dab tsi: Feem ntau ntsev ntsiav tshuaj yog 1g tshuaj uas muaj kwv yees li 300 mus rau 400 milligrams ntawm sodium.
Ua kom pom qhov no, electrolyte ntsev yog dab tsi?
Electrolyte yog lub sij hawm kho mob rau a ntsev los yog ion nyob rau hauv cov ntshav los yog lwm yam dej hauv lub cev uas nqa tus nqi. Thaum sodium chloride los yog rooj ntsev yog ntxiv rau dej, piv txwv li, lub ntsev dissolves thiab lov rau hauv nws cov khoom ions sodium (Na +) thiab chloride (Cl-).
Cov ntsev puas cuam tshuam nrog cov hauv paus?
Yog, xws li cov tshuaj tiv thaiv muaj ntau heev nyob rau hauv yooj yim inorganic chemistry. (1) Muaj zog puag tau yooj yim teb nrog rau ntsev ntawm qaug zog puag thiab tshem nws. Lwm yam alkalis (muaj zog hauv paus ) zoo li NaOH thiab KOH kuj tau yooj yim tso ammonia ntawm kev ua kom sov nrog ammonium ntsev.
Pom zoo:
Dab tsi yog lub npe ntsev tsim los ntawm neutralization ntawm hydrochloric acid thiab sodium hydroxide?
Kev piav qhia: Cov tshuaj tiv thaiv ntawm sodium hydroxide (NaOH) thiab hydrochloric acid (HCl) yog cov tshuaj tiv thaiv nruab nrab uas ua rau muaj ntsev, sodium chloride (NaCl), thiab dej (H2O). Nws yog ib qho tshuaj tiv thaiv exothermic
Dab tsi yog qhov tseem ceeb ntawm cov ntsev yaj hauv dej hiav txwv?

Ntsev nyob rau hauv dej hiav txwv los ntawm pob zeb nyob rau hauv av. Cov nag uas ntog rau hauv av muaj qee cov pa roj carbon dioxide yaj los ntawm cov huab cua ib puag ncig. Qhov no ua rau cov dej nag ua rau acidic me ntsis vim yog carbonic acid (uas tsim los ntawm carbondioxide thiab dej)
Dab tsi yog ntsev ntawm daim duab hauv daim iav dav hlau?

S.A.L.T. Yog daim duab pem hauv ntej, lossis qab daim iav. Puas yog daim duab ze dua lossis deb dua ntawm daim iav dua li qhov khoom
Puas yog NaHS yog ntsev ntsev?

Acid ntsev Cov ntsev tseem muaj hydrogen atom los ntawm cov kua qaub uas tuaj yeem hloov tau los ntawm cov hlau ions ntxiv. Piv txwv xws li: NaHSO4, NaHCO3 thiab NaHS 3. Basic ntsev Cov ntsev muaj cov hydroxides ua ke nrog cov hlau ions thiab cov ions tsis zoo los ntawm cov kua qaub
Puas yog NaH2PO3 yog ntsev ntsev?

Na2HPO3 yog tsim thaum ob lub acidic hydrogens hloov los ntawm sodium. Nws tsis muaj acidic hydrogen. Yog li, nws yog ib txwm sodium ntsev ntawm phosphorus acid. Tab sis NaH2PO3 yog cov ntsev ntsev vim nws tseem muaj ib qho acidic lossis hloov tau hydrogen hauv nws cov muaj pes tsawg leeg
