
Video: Yuav ua li cas daim ntawv cog lus nyob rau hauv ib tug quaternary qauv?

2024 Tus sau: Miles Stephen | [email protected]. Kawg hloov kho: 2023-12-15 23:36
Lub quaternary qauv ntawm cov protein yog kev sib koom ua ke ntawm ntau cov protein chains lossis subunits rau hauv kev sib koom ua ke. Txhua lub subunits muaj nws tus kheej thawj, theem nrab, thiab tertiary qauv . Cov subunits yog tuav ua ke los ntawm hydrogen bonds thiab van der Waals rog ntawm nonpolar sab chains.
Nyob rau hauv no hais txog, yuav ua li cas yog lub quaternary qauv tuav ua ke?
Quaternary qauv yog tuav ua ke los ntawm noncovalent bonds ntawm complementary nto hydrophobic thiab hydrophilic cheeb tsam ntawm lub polypeptide subunits. Tsis tas li ntawd, acidic thiab yooj yim sab chains tuaj yeem tsim kev sib txuas ntsev.
puas quaternary qauv muaj covalent bonds? quaternary qauv piav txog kev sib cuam tshuam, los ntawm kev qaug zog daim ntawv cog lus , ntawm polypeptide sub-units. covalent bonds . Hauv qhov sib piv, lwm pab pawg hais tias muaj protein ntau quaternary qauv yog tias nws muaj tsawg kawg yog ob txoj kev ywj pheej uas tau tuav ua ke los ntawm cov tsis- covalent bonds.
Ntxiv mus, hom ntawv cog lus twg yog tsim nyob rau hauv lub quaternary qauv ntawm cov protein?
Quaternary qauv: Protein yog hais tias nyob rau hauv quaternary qauv yog hais tias lawv muaj ob los yog ntau tshaj polypeptide chains koom ua ke los ntawm cov rog uas tsis yog. covalent bond . Lub zog uas stabilize cov qauv no hydrogen daim ntawv cog lus thiab electrostatic bond.
Dab tsi yog quaternary qauv hauv biology?
Protein quaternary qauv yog tus naj npawb thiab kev npaj ntawm ntau folded protein subunits nyob rau hauv ib tug multi-subunit complex. Nws suav nrog cov koom haum los ntawm qhov yooj yim dimers mus rau homooligomers loj thiab cov complexes nrog cov lus piav qhia lossis cov lej sib txawv ntawm cov subunits.
Pom zoo:
Yuav ua li cas yog daim ntawv cog lus sib txawv ntawm daim ntawv cog lus ionic?
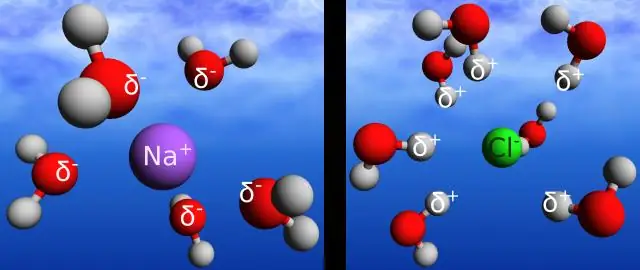
Qhov sib txawv ntawm cov ionic thiab covalent daim ntawv cog lus yog tias covalent daim ntawv cog lus yog tsim thaum ob lub atoms sib koom electrons. Ionic bonds yog lub zog uas tuav ua ke electrostatic zog ntawm kev nyiam ntawm cov ions oppositely them. Ionic bonds muaj qhov sib txawv electronegativity ntau dua lossis sib npaug li 2
Dab tsi yog qhov txawv ntawm daim ntawv cog lus lub zog thiab daim ntawv cog lus dissociation zog?
Qhov sib txawv tseem ceeb ntawm kev sib txuas ntawm lub zog thiab lub zog bonddissociation yog tias lub zog sib txuas yog qhov nruab nrab ntawm lub zog xav tau los rhuav tshem tag nrho cov kev sib txuas ntawm tib ob hom atoms nyob rau hauv ib qho chaw sib txuas whereas daim ntawv cog lus dissociation zog yog tus nqi ntawm cov hluav taws xob xav tau los rhuav tshem ib qho kev sib koom ua ke inhomolysis
Puas yog daim ntawv cog lus hydrogen zoo ib yam li daim ntawv cog lus?
Hydrogen daim ntawv cog lus yog lub npe muab rau electrostatic kev sib cuam tshuam ntawm tus nqi zoo ntawm hydrogen atom thiab tus nqi tsis zoo ntawm oxygen atom ntawm ib qho chaw nyob sib ze. Covalent daim ntawv cog lus yog qhov sib cuam tshuam electrostatic ntawm ob lub atom hauv tib lub molecule
Yuav ua li cas yog lub sum ntawm cov atomic masses ntawm tag nrho cov atoms nyob rau hauv ib tug qauv rau ib tug compound?

Cov mis loj ntawm ib yam khoom yog qhov sib npaug ntawm qhov nruab nrab atomic masses ntawm txhua lub atom sawv cev hauv cov tshuaj formula thiab qhia nyob rau hauv atomic mass units. Cov mis loj ntawm covalent compound tseem hu ua molecular mass
Yuav ua li cas koj sau txoj kab nqes nqes intercept daim ntawv nyob rau hauv tus qauv daim ntawv?
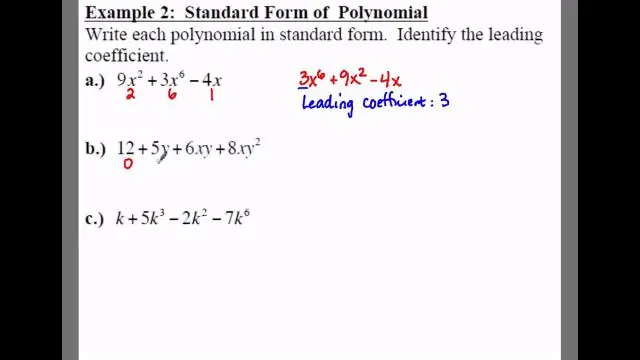
Standard form yog lwm txoj hauv kev los sau daim ntawv nqes hav-intercept (as opposed to y=mx+b). Nws sau li Ax+By=C. Koj tuaj yeem hloov daim ntawv nqes hav-intercept rau cov qauv zoo li no: Y=-3/2x + 3. Tom ntej no, koj cais qhov y-intercept (qhov no nws yog 2) zoo li no: Ntxiv 3/2x rau txhua sab ntawm kab zauv kom tau qhov no: 3/2x + y = 3
