Cov txheej txheem:

Video: Yuav ua li cas koj xam enthalpy hloov nyob rau hauv chemistry?

2024 Tus sau: Miles Stephen | [email protected]. Kawg hloov kho: 2023-12-15 23:36
Siv cov mis ∆H = m x s x ∆T los daws.
Thaum koj muaj m, qhov loj ntawm koj cov reactants, s, qhov tshwj xeeb kub ntawm koj cov khoom, thiab ∆T, qhov kub thiab txias hloov los ntawm koj cov tshuaj tiv thaiv, koj tau npaj los nrhiav qhov enthalpy ntawm cov tshuaj tiv thaiv. Tsuas yog ntsaws koj qhov tseem ceeb rau hauv cov qauv ∆H = m x s x ∆T thiab muab faib los daws.
Hais txog qhov no, koj ua li cas xam lub zog hloov hauv chemistry?
Txhawm rau xam qhov hloov pauv hluav taws xob rau qhov tshuaj tiv thaiv:
- ntxiv ua ke lub zog ntawm daim ntawv cog lus rau txhua daim ntawv cog lus hauv cov reactants - qhov no yog 'zog hauv'
- ntxiv ua ke lub zog ntawm daim ntawv cog lus rau txhua daim ntawv cog lus hauv cov khoom - qhov no yog 'zog tawm'
- zog hloov = zog hauv - zog tawm.
Ib tug kuj nug, Q MC _firxam_#8710 yog dab tsi; T siv rau? Q = mc∆T . Q = tshav kub zog (Joules, J) m = loj ntawm ib yam khoom (kg) c = tshwj xeeb kub (units J / kg∙K) ∆ yog lub cim txhais tias "kev hloov hauv"
Tib neeg kuj nug, qhov kev hloov pauv hauv enthalpy rau cov tshuaj tiv thaiv tshuaj yog dab tsi?
Rau ib tshuaj tiv thaiv , cov enthalpy ntawm tshuaj tiv thaiv (ΔHrxn) yog qhov sib txawv hauv enthalpy nruab nrab ntawm cov khoom thiab reactants; units ntawm ΔHrxn yog kilojoules ib mole. Rov qab a tshuaj tiv thaiv thim rov qab kos npe ntawm ΔHrxn.
Koj txhais li cas enthalpy?
Enthalpy yog ib qho khoom siv thermodynamic ntawm ib qho system. Nws yog tus lej ntawm lub zog sab hauv ntxiv rau cov khoom ntawm lub siab thiab ntim ntawm lub cev. Nws qhia txog lub peev xwm ua haujlwm tsis yog siv tshuab thiab lub peev xwm tso tawm cua sov. Enthalpy yog denoted li H; tshwj xeeb enthalpy npe h.
Pom zoo:
Yuav ua li cas koj xam ua nyob rau hauv voltage?

Lub peev xwm built-in (ntawm 300 K) sib npaug fi = kT/q ln(1016 x 9 x 1017/ni2) = 0.77 V, siv kT/q = 25.84 mV thiab ni = 1010 cm-3. Lub peev xwm ua tau (ntawm 100 ° C) sib npaug fi = kT/q ln(1016 x 9 x 1017/ni2) = 0.673 V, siv kT/q = 32.14 mV thiab ni = 8.55 x 1011 cm-3 (los ntawm Piv txwv 20)
Yuav ua li cas koj xam Pearson khoom lub sij hawm correlation nyob rau hauv SPSS?
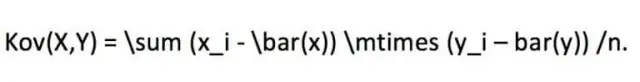
Txhawm rau khiav qhov sib txawv ntawm Pearson Correlation, nyem Tshawb xyuas> Correlate> Bivariate. Xaiv qhov sib txawv Qhov siab thiab qhov hnyav thiab txav mus rau hauv lub thawv Variables. Hauv cheeb tsam Correlation Coefficients, xaiv Pearson. Nyob rau hauv qhov kev ntsuam xyuas qhov tseem ceeb, xaiv qhov koj xav tau qhov tseem ceeb xeem, ob-tailed los yog ib-tailed
Yuav ua li cas koj xam cov kev hloov nyob rau hauv latitude?

Xam cov kev hloov ntawm Latitude thiab Longitude. Yog hais tias lub latitudes nyob rau hauv txawv hemispheres ces ntxiv. Yog tias cov latitudes nyob hauv tib hemispheres ces rho tawm. 60 ° 36' yog qhov hloov pauv inlatitude
Yuav ua li cas koj xam rov qab nyob rau hauv distillation?

Txiav txim siab qhov feem pua ntawm cov distillation rov qab los ntawm kev faib cov kua distilled rov qab los ntawm cov vapor los ntawm tus nqi qub ntawm cov kua. Qhov no qhia koj seb qhov feem pua ntawm cov kua qub tau muab tso rau hauv cov tshuaj muaj zog dua
Yuav ua li cas koj xam cov kev hloov nyob rau hauv equilibrium?

Equilibrium yog txiav txim los ntawm kev ntxiv 'Initial' thiab 'Hloov ua ke. Yog tias x = 1.78 ces [C2H4]Eq yog qhov tsis zoo, uas yog tsis yooj yim sua, yog li, x yuav tsum sib npaug 0.098
