
Video: Yuav ua li cas koj paub yog hais tias ib tug txheej txheem yog spontaneous?

2024 Tus sau: Miles Stephen | [email protected]. Kawg hloov kho: 2023-12-15 23:36
Los ntawm kev xav txog ob yam no, peb tuaj nrog Gibbs Free Energy sib npaug los kwv yees yog ib qho tshuaj tiv thaiv yuav mus ntxiv tus kheej los tsis. Yog lub Gibbs Dawb Zog yog qhov tsis zoo, ces cov tshuaj tiv thaiv yog tus kheej , thiab yog nws yog qhov zoo, ces nws yog nonspontaneous.
Tom qab ntawd, dab tsi ua rau tus txheej txheem spontaneous?
A txheej txheem spontaneous yog lub sij hawm-evolution ntawm ib tug system nyob rau hauv uas nws tso tawm lub zog dawb thiab nws txav mus rau ib tug qis dua, ntau thermodynamically ruaj khov lub zog. Rau cov xwm txheej uas muaj kev sib cais uas tsis muaj lub zog hloov pauv nrog ib puag ncig, cov txheej txheem spontaneous yog tus cwj pwm los ntawm kev nce hauv entropy.
Tom qab ntawd, lo lus nug yog, qhov twg ob lub thermodynamic kom txiav txim siab seb tus txheej txheem puas yog tus kheej? Nyob rau hauv qhov kub thiab txias, qhov sib txawv ntawm G (Gibbs Zog) ntawm cov khoom thiab cov reactants txiav txim spontaneity . Yog tias delta G yog qhov tsis zoo, qhov txheej txheem yog spontaneous . Yog tias delta G yog qhov zoo, nws yog tsis spontaneous.
Ntawm no, dab tsi ua rau cov tshuaj tiv thaiv spontaneous lossis Nonspontaneous?
Kev tshwm sim tshwm sim . Cov tshuaj tiv thaiv yog qhov zoo thaum lawv ua rau txo qis hauv enthalpy thiab nce hauv entropy ntawm lub system. Thaum ob qho tib si ntawm cov xwm txheej no tau ntsib, cov tshuaj tiv thaiv tshwm sim lawm. A cov tshuaj tiv thaiv tsis raws cai yog a tshuaj tiv thaiv uas tsis nyiam tsim cov khoom ntawm cov txheej txheem muab.
Qhov unit ntawm entropy yog dab tsi?
The SI chav tsev rau Entropy (S) yog Joules per Kelvin (J/K). Tus nqi zoo dua ntawm entropy txhais tau hais tias ib qho tshuaj tiv thaiv yuav tshwm sim spontaneously.
Pom zoo:
Yuav ua li cas koj paub yog hais tias ib tug compound yog molecular?
Mixed Ionic / Molecular Compound Naming. Thaum naming cov tebchaw, thawj qhov uas koj yuav tsum tau ua yog txiav txim siab seb lub compound yog ionic lossis molecular. Saib cov ntsiab lus hauv qhov sib xyaw. * Ionic tebchaw yuav muaj ob qho tib si hlau thiab tsis yog hlau, los yog tsawg kawg yog ib tug polyatomic ion. * Molecular compounds yuav tsuas muaj cov hlau tsis muaj hlau
Yuav ua li cas koj thiaj paub yog hais tias ib tug function tsis yog ib tug function?
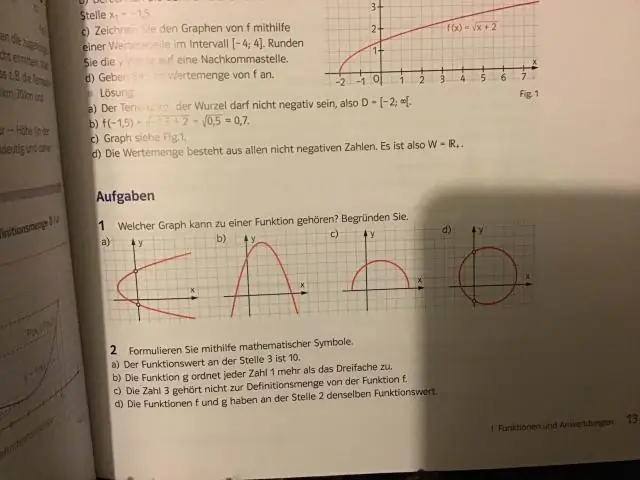
Kev txiav txim siab seb qhov kev sib raug zoo yog qhov ua haujlwm ntawm daim duab yog qhov yooj yim los ntawm kev siv cov kab ntsug. Yog tias txoj kab ntsug hla qhov kev sib raug zoo ntawm daim duab ib zaug hauv txhua qhov chaw, qhov kev sib raug zoo yog qhov ua haujlwm. Txawm li cas los xij, yog tias txoj kab ntsug hla qhov kev sib raug ntau dua ib zaug, qhov kev sib raug zoo tsis muaj nuj nqi
Koj tuaj yeem ua li cas yog tias koj tsis paub tias txheej twg yog nyob rau hauv cov txheej txheem rho tawm?

Koj tuaj yeem ua li cas yog tias koj tsis paub tias txheej twg yog nyob rau hauv cov txheej txheem rho tawm? Tso dej me me rau hauv lub caj dab ntawm qhov sib cais funnel. Ua tib zoo saib nws: yog tias nws tseem nyob hauv txheej txheej sab saud, txheej txheej ntawd yog txheej dej
Dab tsi yog txheej txheem spontaneous thiab non-spontaneous txheej txheem?

Cov txheej txheem spontaneous yog ib qho uas tshwm sim yam tsis muaj kev cuam tshuam ntawm sab nraud. Ib txoj kev uas tsis yog tshwm sim yuav tsis tshwm sim yog tias tsis muaj kev cuam tshuam ntawm sab nraud
Yuav ua li cas ib tug geologist yuav qhia tau hais tias ib tug quav yog ib tug syncline thiab anti-line?

Geologic Structures (Part 5) Anticlines yog folds nyob rau hauv uas txhua ib nrab ntawm cov quav dips deb ntawm lub crest. Synclines yog folds nyob rau hauv uas txhua ib nrab ntawm cov quav dips mus rau lub trough ntawm lub quav. Koj tuaj yeem nco qab qhov sib txawv los ntawm kev ceeb toom tias anticlines tsim "A" zoo, thiab synclines tsim hauv qab ntawm "S."
