Video: Yuav ua li cas koj xam qhov nruab nrab atomic mass ntawm strontium?

2024 Tus sau: Miles Stephen | [email protected]. Kawg hloov kho: 2023-12-15 23:36
Yog li ntawd, peb xam nws los ntawm kev noj qhov hnyav pawg ntawm txhua tus isotopes thiab ntxiv rau lawv ua ke. Yog li, rau thawj pawg , peb yuav muab 0.50% ntawm 84 (amu - atomic loj units) = 0.042 amu, thiab ntxiv rau 9.9% ntawm 86 amu = 8.51 amu, thiab lwm yam.
Tom qab ntawd, ib tug kuj yuav nug, ua li cas koj xam qhov nruab nrab atomic mass?
Xam qhov nruab nrab Atomic Mass Cov nruab nrab atomic mass ntawm ib lub ntsiab yog cov sum ntawm cov pawg ntawm nws cov isotopes, txhua qhov sib npaug los ntawm nws qhov kev nplua nuj (tus lej suav nrog feem pua ntawm atoms ntawm cov khoom uas yog ntawm ib qho isotope muab). Qhov nruab nrab atomic mass = f1M1 + f2M2 +…
Kuj Paub, peb hom ntaub ntawv dab tsi xav tau los xam qhov nruab nrab atomic mass? Tus naj npawb ntawm Isotopes uas muaj rau lub ntsiab. Cov Atomic Tus nab npawb rau lub ntsiab. Qhov feem pua ntawm txhua qhov isotope.
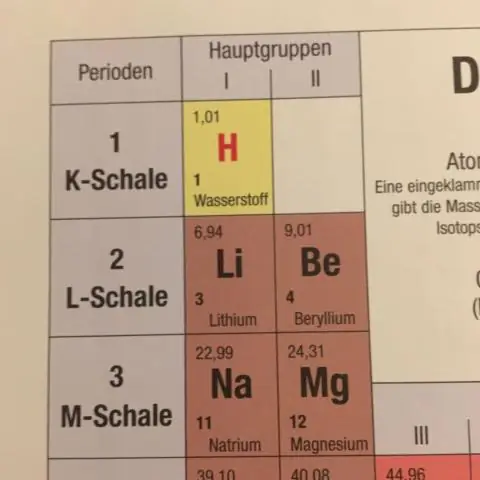
Ib tug kuj yuav nug, dab tsi tus nqi yog ze tshaj rau atomic loj ntawm strontium?
Strontium . Hauv nws daim ntawv tshaj tawm xyoo 1961, Pawg Neeg Saib Xyuas tau pom zoo Ar(Sr) = 87.62 raws li cov pawg -spectrometric kev txiav txim siab ntawm Nier. Qhov no tus nqi tau hloov kho rau Ar(Sr) = 87.62(1) hauv 1969 thiab nws tseem tsis tau hloov txij li thaum.
Dab tsi muaj qhov loj ntawm 1 amu?
Lub atomic mass unit (symbolized AMU lossis amu) yog txhais raws li qhov tseeb 1/12 qhov loj ntawm ib qho atom ntawm carbon-12. Cov pa roj carbon-12 (C-12) atom muaj rau protons thiab rau neutrons hauv nws cov nucleus. Hauv cov ntsiab lus tsis meej, ib qho AMU yog qhov nruab nrab ntawm qhov proton so mass thiab neutron so mass.
Pom zoo:
Qhov nruab nrab atomic mass ntawm ib atom yog dab tsi?

Qhov nruab nrab atomic huab hwm coj ntawm ib lub caij yog qhov sib npaug ntawm cov huab hwm coj ntawm nws cov isotopes, txhua qhov sib npaug los ntawm nws qhov kev nplua nuj (cov zauv feem cuam tshuam nrog feem pua ntawm cov atoms ntawm lub caij uas yog ntawm cov isotope muab). Qhov nruab nrab atomic mass = f1M1 + f2M2 +
Yuav ua li cas koj pom qhov nruab nrab thiab nruab nrab ntawm daim duab peb sab?

Txhawm rau nrhiav qhov chaw nruab nrab ntawm daim duab peb sab, nws yog qhov yooj yim tshaj plaws los kos tag nrho peb qhov nruab nrab thiab nrhiav lawv qhov kev sib tshuam. Txhawm rau kos qhov nruab nrab ntawm daim duab peb sab, ua ntej nrhiav qhov nruab nrab ntawm ib sab ntawm daim duab peb sab. Kos ib kab ntu uas txuas qhov taw tes no mus rau qhov rov qab vertex
Qhov nruab nrab nruab nrab ntawm lub ntiaj teb yog dab tsi?
Lub teb chaws qhov ntsuas kub yog 2.91 ° C (5.24 ° F) saum toj no 1961-1990 qhov nruab nrab, tawg cov ntaub ntawv yav dhau los tau teev tseg hauv 2013 los ntawm 0.99 ° C (1.78 ° F)
Koj pom qhov nruab nrab thiab nruab nrab hauv tableau li cas?
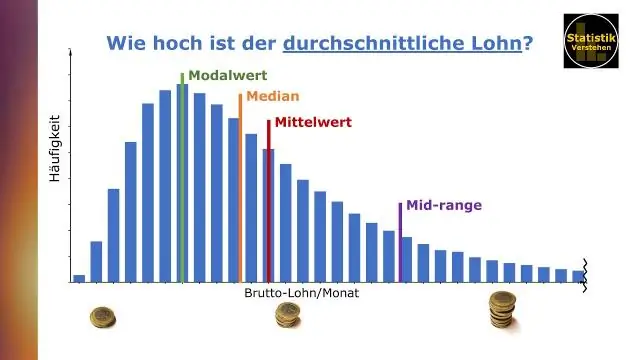
Kev sib txuas lus cov ntaub ntawv nrog Tableau los ntawm Ben Jones Qhov nruab nrab (lossis nruab nrab) yog txiav txim siab los ntawm kev suav tag nrho cov txiaj ntsig hauv cov ntaub ntawv teev thiab faib los ntawm tus lej ntawm qhov tseem ceeb. Qhov nruab nrab yog tus nqi nruab nrab hauv cov ntaub ntawv teev nyob rau hauv uas qhov tseem ceeb tau muab tso rau hauv kev txiav txim ntawm qhov loj
Koj ua li cas sib npaug redox cov tshuaj tiv thaiv hauv acidic thiab nruab nrab nruab nrab?

Acid Conditions Solution. Kauj Ruam 1: Sib cais ib nrab cov lus teb. Kauj Ruam 2: Ntsuas cov ntsiab lus uas tsis yog O thiab H. Kauj Ruam 3: Ntxiv H2O kom sib npaug ntawm cov pa. Kauj Ruam 4: Sib npaug hydrogen los ntawm kev ntxiv protons (H +). Kauj Ruam 5: Ntsuas tus nqi ntawm txhua qhov sib npaug nrog electrons. Kauj Ruam 6: Ntsuas cov tshuaj tiv thaiv kom cov electrons sib npaug
