
Video: Qhov nruab nrab atomic mass ntawm ib atom yog dab tsi?

2024 Tus sau: Miles Stephen | [email protected]. Kawg hloov kho: 2023-12-15 23:36
Cov nruab nrab atomic mass ntawm ib lub caij yog qhov sum ntawm pawg ntawm nws cov isotopes, txhua qhov sib npaug los ntawm nws qhov kev nplua nuj (tus lej suav nrog feem pua ntawm atoms ntawm qhov ntawd keeb uas yog ntawm ib qho isotope muab). Qhov nruab nrab atomic mass = f1M1 + f2M2 +…
Tom qab ntawd, ib tug kuj yuav nug, yuav ua li cas yog lub atomic loj ntawm ib lub ntsiab xam?
Rau xam tus atomic loj ntawm ib leeg atom ntawm ib keeb , add rau pawg ntawm protons thiab neutrons. Piv txwv: Nrhiav cov atomic loj ntawm ib qho isotope ntawm carbon uas muaj 7 neutrons. Koj tuaj yeem pom los ntawm cov lus ntu ntu uas carbon muaj atomic tus naj npawb ntawm 6, uas yog nws tus naj npawb ntawm protons.
Tsis tas li ntawd, puas yog atomic mass zoo ib yam li atomic hnyav? Atomic loj (mib) yog pawg ntawm ib atom . Ib leeg atom muaj cov txheej txheem ntawm protons thiab neutrons, yog li cov pawg yog unequivocal (yuav tsis hloov) thiab yog cov sum ntawm tus naj npawb ntawm protons thiab neutrons nyob rau hauv lub atom . Atomic hnyav yog qhov hnyav nruab nrab ntawm qhov pawg ntawm tag nrho cov atoms ntawm ib lub caij, raws li kev nplua nuj ntawm isotopes.
Ua li no, koj ua li cas xam qhov nruab nrab atomic loj ntawm carbon?
Rau nrhiav tus nruab nrab atomic mass , koj coj ib tug xov tooj ntawm atoms , nrhiav tag nrho pawg ntawm txhua isotope, thiab tom qab ntawd faib tag nrho pawg ntawm tag nrho cov atoms los ntawm tag nrho cov naj npawb ntawm atoms . Xav tias koj muaj, hais, 10 000 atoms ntawm carbon . Tom qab ntawd koj muaj 9893 atoms ntawm 12C thiab 107 atoms ntawm 13C.
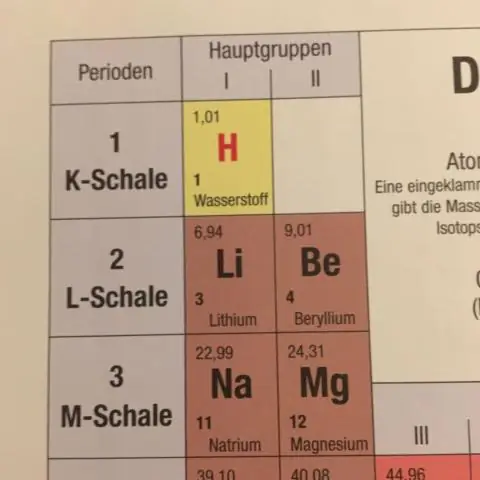
Qhov nruab nrab atomic loj ntawm titanium nyob rau hauv lub ntiaj teb yog dab tsi?
Isotope: 46Ti Muaj peev xwm: 75.200% Pawg 45.95263 Amu Isotope: 48Ti Kev nplua nuj: 12.300% Pawg 47.94795 Amu Isotope: 50Ti Kev nplua nuj: 12.500% Pawg : 49.94479 Amu.
Pom zoo:
Yuav ua li cas koj xam qhov nruab nrab atomic mass ntawm strontium?
Yog li, peb xam nws los ntawm kev noj qhov hnyav ntawm txhua tus isotopes thiab ntxiv rau lawv ua ke. Yog li, rau thawj pawg, peb yuav muab 0.50% ntawm 84 (amu - atomic mass units) = 0.042 amu, thiab ntxiv rau 9.9% ntawm 86 amu = 8.51 amu, thiab lwm yam
Yuav ua li cas koj pom qhov nruab nrab thiab nruab nrab ntawm daim duab peb sab?

Txhawm rau nrhiav qhov chaw nruab nrab ntawm daim duab peb sab, nws yog qhov yooj yim tshaj plaws los kos tag nrho peb qhov nruab nrab thiab nrhiav lawv qhov kev sib tshuam. Txhawm rau kos qhov nruab nrab ntawm daim duab peb sab, ua ntej nrhiav qhov nruab nrab ntawm ib sab ntawm daim duab peb sab. Kos ib kab ntu uas txuas qhov taw tes no mus rau qhov rov qab vertex
Qhov nruab nrab nruab nrab ntawm lub ntiaj teb yog dab tsi?
Lub teb chaws qhov ntsuas kub yog 2.91 ° C (5.24 ° F) saum toj no 1961-1990 qhov nruab nrab, tawg cov ntaub ntawv yav dhau los tau teev tseg hauv 2013 los ntawm 0.99 ° C (1.78 ° F)
Koj pom qhov nruab nrab thiab nruab nrab hauv tableau li cas?
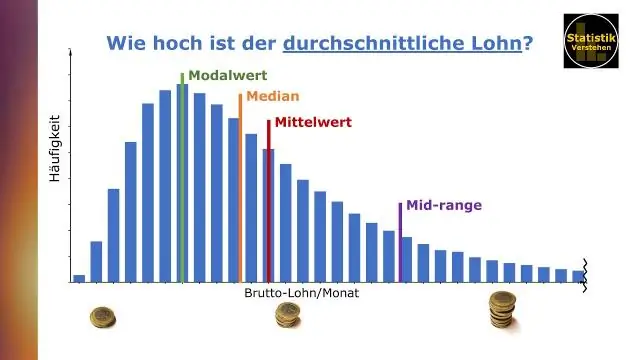
Kev sib txuas lus cov ntaub ntawv nrog Tableau los ntawm Ben Jones Qhov nruab nrab (lossis nruab nrab) yog txiav txim siab los ntawm kev suav tag nrho cov txiaj ntsig hauv cov ntaub ntawv teev thiab faib los ntawm tus lej ntawm qhov tseem ceeb. Qhov nruab nrab yog tus nqi nruab nrab hauv cov ntaub ntawv teev nyob rau hauv uas qhov tseem ceeb tau muab tso rau hauv kev txiav txim ntawm qhov loj
Dab tsi yog qhov txawv ntawm instantaneous thiab nruab nrab ceev dab tsi yog qhov piv txwv loj tshaj ntawm kev ceev ceev?
Qhov nruab nrab ceev yog qhov ceev nruab nrab ntawm lub sijhawm. Instantaneous ceev yuav yog qhov ceev txhua qhov muab tam sim ntawd hauv lub sijhawm ntawd, ntsuas nrog lub ntsuas ntsuas lub sijhawm
