
Video: Puas yog ib qho tsis muaj polar molecule puas muaj hydrogen bonding?
2024 Tus sau: Miles Stephen | [email protected]. Kawg hloov kho: 2023-12-15 23:36
Yog tias molecule yog tsis muaj polar , ces tsis muaj dipole-dipole kev sib cuam tshuam los yog hydrogen bonding tau tshwm sim thiab tsuas yog ua tau intermolecular quab yuam yog lub zog van der Waals tsis muaj zog.
Tsuas yog li ntawd, cov molecules nonpolar puas tsim tau hydrogen bonds?
Dej yog Polar The hydrogen thiab oxygen atoms hauv dej molecules daim ntawv polar covalent daim ntawv cog lus . Cov electrons sib koom siv sij hawm ntau dua nrog cov pa atom dua li lawv ua nrog hydrogen atoms. Hydrogen bonds tsis tau yooj yim tsim nrog tsis muaj polar tshuaj xws li roj thiab roj (Daim duab 1).
Tom qab, lo lus nug yog, hom molecules yuav tso tawm hydrogen bonding? Hydrogen daim ntawv cog lus tsuas yog tsim los ntawm peb lub ntsiab lus electronegative heev - fluorine, oxygen thiab nitrogen. Yog li ntawd, hydrogen bonding tsuas yog ua tau nyob rau hauv cov tebchaw uas cov hydrogen atom ncaj qha ua phooj ywg rau fluorine, oxygen lossis nitrogen.
Tsis tas li ntawd, puas hydrogen bonds tsuas yog tshwm sim polar molecules?
Cov hydrogen daim ntawv cog lus hauv polar molecules tshwm sim xwb hauv cov tshuaj uas muaj hydrogen bonded rau N, O, los yog F. H atom yog attracted rau ib feem tsis zoo nqi ntawm ib tug N, O, los yog F atom nyob rau hauv lwm yam molecule . Cov hydrogen daim ntawv cog lus yog ib qho attraction tab sis tsis muaj tseeb tshuaj daim ntawv cog lus xws li ionic lossis covalent daim ntawv cog lus.
Dab tsi ntawm daim ntawv cog lus yog dej?
Dej yog ib tug polar molecule A dej molecule yog tsim thaum ob atoms ntawm hydrogen daim ntawv cog lus covalently nrog ib tug atom ntawm oxygen. Hauv covalent daim ntawv cog lus electrons sib koom ntawm atoms. Hauv dej kev sib koom tsis sib npaug. Cov pa oxygen atom nyiam cov electrons ntau dua li cov hydrogen.
Pom zoo:
Polar molecules puas tiv thaiv cov tsis muaj polar molecules?

Polar molecules (nrog +/- tsub) yog nyiam rau cov dej molecules thiab yog hydrophilic. Nonpolar molecules yog repelled los ntawm dej thiab tsis yaj hauv dej; yog hydrophobic
Vim li cas lub pob thiab lo qauv ntawm lub molecule yog ib qho duab tsis muaj tseeb?
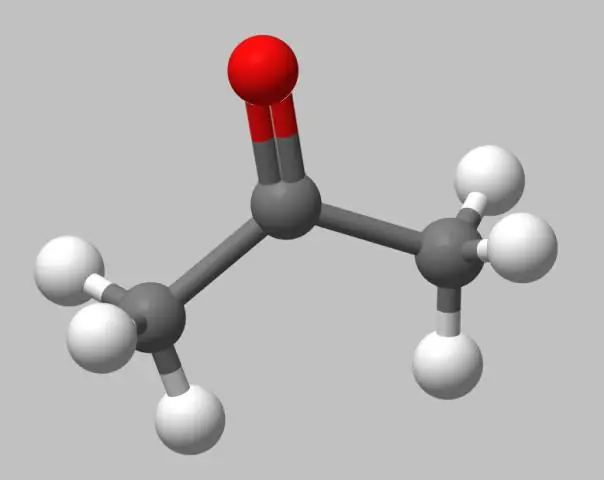
Pob-thiab-stick qauv. Cov qauv pob-thiab-loj tsis yog qhov tseeb raws li qhov chaw ntim cov qauv, vim hais tias cov atoms tau piav qhia raws li cov spheres ntawm radii me dua lawv cov van der Waals radii. Txawm li cas los xij, kev sib koom ua ke tau yooj yim pom vim tias cov ntawv cog lus tau qhia meej meej tias yog cov nplaum
Leej twg yog tus qauv ntawm ib qho tsis muaj polar molecule uas muaj cov ntawv cog lus nonpolar?

(1), (3) H2O thiab NH3 yog cov molecules uas muaj polar covalent bonds, tab sis lawv cov khoom siv hluav taws xob tsis sib luag. (4) H2 yog ib tug nonpolar molecule uas muaj ib tug symmetrical tis ntawm electrons, tab sis cov nyiaj ntawm cov hydrogen atoms yog nonpolar covalent
Puas yog fluoromethane muaj hydrogen bonding?

Tsis tas li ntawd, cov molecule tsis muaj hydrogen atoms sib txuas rau nitrogen, oxygen, lossis fluorine; txiav txim tawm hydrogen bonding. Thaum kawg, muaj ib qho dipole tsim los ntawm qhov sib txawv ntawm electronegativity ntawm carbon thiab fluorine atoms. Qhov no txhais tau tias fluoromethane molecule yuav muaj zog dipole-dipole
Puas yog n2 muaj hydrogen bonding?

Nitrogen (N2) yog ib qho tsis-polar molecule thiab tsuas yog tsim nyob rau lub lim tiam London dispersion rog ntawm nws cov molecules. Dej yog ib qho polar molecule uas tsim muaj zog hydrogen bonds ntawm nws cov molecules. Yog tias N2 tuaj yeem tsim hydrogen bonds nrog dej, nws yuav yog dej soluble heev
