
Video: Dab tsi yog daim ntawv cog lus kaum rau saws?

2024 Tus sau: Miles Stephen | [email protected]. Kawg hloov kho: 2023-12-15 23:36
Cov saws puab maximizes lub daim ntawv cog lus cov ces kaum ntawm ib leeg ib khub thiab lwm yam atoms nyob rau hauv lub molecule. Cov khub ib leeg yog nyob rau hauv ib txoj hauj lwm equatorial muab 120 thiab 90 degree daim ntawv cog lus cov ces kaum , piv rau 90 degree xwb daim ntawv cog lus cov ces kaum yog muab tso rau ntawm txoj hauj lwm axial.
Raws li, qhov twg molecule muaj ib tug seesaw zoo li?
Feem ntau, plaub daim ntawv cog lus rau lub hauv paus atom ua rau tetrahedral lossis, tsawg dua, square planar geometry. Lub seesaw geometry, ib yam li nws lub npe, yog txawv. Nws tshwm sim thaum lub molecule muaj tus lej steric ntawm 5, nrog rau hauv nruab nrab atom raug khi rau 4 lwm atoms thiab 1 leeg ib khub (AX4E hauv AX notation).
Ib sab saum toj no, dab tsi yog daim ntawv cog lus ntawm cov square planar? Teb: Cov daim ntawv cog lus cov ces kaum hauv cov cov duab yuav yog kwv yees li qhov lawv nyob hauv geometrical configuration lawv los ntawm. Square planar daim ntawv cog lus kaum yuav yog 90 degrees.
Ib yam li ntawd, lub kaum sab xis ntawm lub trigonal bipyramidal yog dab tsi?
Trigonal bipyramidal cov tsos mob : tsib atoms nyob ib ncig ntawm lub hauv paus atom; peb hauv ib lub dav hlau nrog daim ntawv cog lus cov ces kaum ntawm 120 ° thiab ob qho tib si ntawm qhov kawg ntawm cov molecule. Octahedral: rau lub atom nyob ib ncig ntawm lub hauv paus atom, tag nrho nrog daim ntawv cog lus cov ces kaum ntawm 90 °.
Puas yog seesaw polar lossis nonpolar?
Nyob rau hauv VSEPR txoj kev xav, tus khub ib leeg yuam cov molecular geometry ntawm SF4 rau hauv cov duab pom. Ob ntawm S-F daim ntawv cog lus tau taw qhia deb ntawm ib leeg, thiab lawv daim ntawv cog lus dipoles tshem tawm. Tab sis lwm ob S-F dipoles yog taw tes "down". Lawv daim ntawv cog lus dipoles tsis tso tseg, yog li cov molecule yog polar.
Pom zoo:
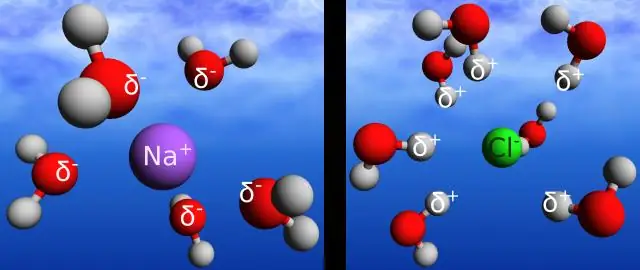
Yuav ua li cas yog daim ntawv cog lus sib txawv ntawm daim ntawv cog lus ionic?
Qhov sib txawv ntawm cov ionic thiab covalent daim ntawv cog lus yog tias covalent daim ntawv cog lus yog tsim thaum ob lub atoms sib koom electrons. Ionic bonds yog lub zog uas tuav ua ke electrostatic zog ntawm kev nyiam ntawm cov ions oppositely them. Ionic bonds muaj qhov sib txawv electronegativity ntau dua lossis sib npaug li 2
Dab tsi yog qhov txawv ntawm daim ntawv cog lus lub zog thiab daim ntawv cog lus dissociation zog?
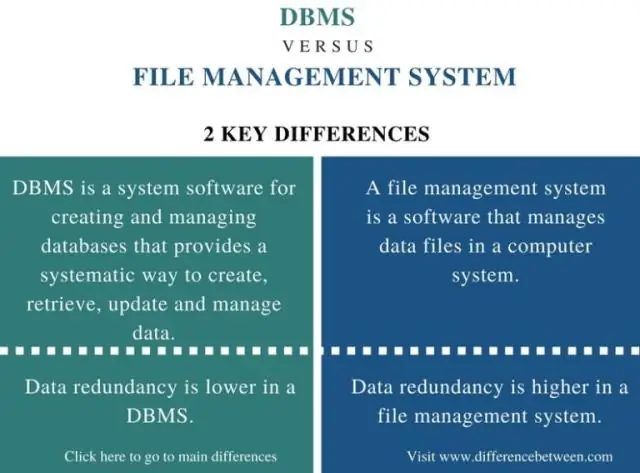
Qhov sib txawv tseem ceeb ntawm kev sib txuas ntawm lub zog thiab lub zog bonddissociation yog tias lub zog sib txuas yog qhov nruab nrab ntawm lub zog xav tau los rhuav tshem tag nrho cov kev sib txuas ntawm tib ob hom atoms nyob rau hauv ib qho chaw sib txuas whereas daim ntawv cog lus dissociation zog yog tus nqi ntawm cov hluav taws xob xav tau los rhuav tshem ib qho kev sib koom ua ke inhomolysis
Puas yog daim ntawv cog lus hydrogen zoo ib yam li daim ntawv cog lus?
Hydrogen daim ntawv cog lus yog lub npe muab rau electrostatic kev sib cuam tshuam ntawm tus nqi zoo ntawm hydrogen atom thiab tus nqi tsis zoo ntawm oxygen atom ntawm ib qho chaw nyob sib ze. Covalent daim ntawv cog lus yog qhov sib cuam tshuam electrostatic ntawm ob lub atom hauv tib lub molecule
Dab tsi yog covalent daim ntawv cog lus rau dummies?

Environmental Science For Dummies Thaum ob lub atoms sib koom ua ke hauv cov ntawv cog lus, lawv tsim cov molecule uas sib koom electrons. Tsis zoo li nyob rau hauv daim ntawv cog lus ionic, ob qho tib si ntawm cov atoms hauv covalent daim ntawv cog lus poob lossis tau txais ib qho hluav taws xob; Hloov chaw, ob lub atoms siv ib khub ntawm cov electrons sib koom
Dab tsi yog CL Al Cl daim ntawv cog lus lub kaum hauv AlCl3?

Lub Cl-Al-CI daim ntawv cog lus lub kaum sab xis yog 116. goin cov nqe lus ntawm ib tug r ib tug nruab nrab qauv 3 nrog ib tug uncertainty ntawm aboutO
