Cov txheej txheem:

Video: Yuav ua li cas tag nrho cov compounds zoo sib xws?
2024 Tus sau: Miles Stephen | [email protected]. Kawg hloov kho: 2023-12-15 23:36
Kev piav qhia: Cov tshuaj zoo li CO2 yog ua los ntawm atoms muab tso ua ke kom ruaj khov molecules. Tag nrho cov Compounds yeej ib txwm ua los ntawm atoms ntawm ntau hom los ua molecules. Cov ntsiab lus thaum lawv yog molecules yeej ib txwm ua los ntawm atoms tib yam hom.
Ua raws li qhov no hauv kev txiav txim siab, cov ntsiab lus thiab cov tebchaw zoo li cas?
A compound muaj cov atom sib txawv cov ntsiab lus chemically ua ke ua ke nyob rau hauv ib tug taag ratio. Ib keeb yog ib hom tshuaj dawb huv ua los ntawm tib hom atom. Cov tshuaj muaj qhov sib txawv cov ntsiab lus nyob rau hauv ib tug taag ratio teem nyob rau hauv ib tug txhais lus los ntawm chemical bonds.
Tsis tas li ntawd, qhov twg tsis yog qhov sib xyaw? Hydrogen gas (H2) yog molecule, tab sis tsis yog compound vim nws tsuas yog ua los ntawm ib lub ntsiab xwb. Dej (H2O) tuaj yeem hu ua molecule lossis a compound vim nws yog tsim los ntawm hydrogen (H) thiab oxygen (O) atoms. Muaj ob lub ntsiab ntawm cov khoom sib txuas uas tuav cov atoms ua ke: covalent thiab ionic / electrovalent bonds.
Ntawm no, dab tsi tuaj yeem muaj cov ntsiab lus thiab cov khoom sib xyaw ua ke?
Elements thiab compounds yog homogeneous nkaus xwb tshuaj thiab lawv muaj ib tug tas mus li muaj pes tsawg leeg thoob plaws. Elements thiab compounds tsis tuaj yeem muab cais rau hauv lawv cov khoom sib xws los ntawm kev siv lub cev. Cov tshuaj thiab kev sib tov yog ua los ntawm kev sib txawv cov ntsiab lus los yog txawv atoms.
4 hom kev sib txuas yog dab tsi?
Muaj plaub hom kev sib txuas, nyob ntawm seb cov atoms muaj pes tsawg leeg tau tuav ua ke:
- molecules tuav ua ke los ntawm covalent bonds.
- ionic compounds tuav ua ke los ntawm ionic bonds.
- intermetallic compounds tuav ua ke los ntawm metallic bonds.
- qee qhov complexes tuav ua ke los ntawm kev sib koom tes covalent bonds.
Pom zoo:
Vim li cas thiaj li yuav tsum muaj qhov sib npaug ntawm tag nrho 3 los txhawb kev loj hlob zoo tshaj plaws?

Dab tsi cais ib lub qab ntug los ntawm lwm qhov? Yuav tsum muaj kev sib npaug kom cov av khaws cov dej thiab cia dej ntws tawm ntawm nws, yog tias cov av yog xuab zeb hnyav, dej yuav yooj yim ntws tawm ntawm nws los yog cov av yog av nplaum-hnyav ces dej tsis tuaj yeem nkag los ntawm nws. thiab cov cag ntoo yuav tawm tsam
Puas yog ob txoj kab sib luag zoo sib xws lossis tsis sib xws?

Yog tias ob qhov sib npaug piav txog kab sib luag, thiab yog li cov kab uas tsis sib tshuam, qhov system yog ywj siab thiab tsis sib haum. Yog hais tias ob qhov sib npaug piav txog tib kab, thiab yog li cov kab uas hla tus lej tsis kawg, lub kaw lus yog nyob ntawm thiab sib xws
Koj tuaj yeem ua pov thawj li cas 2 daim duab peb sab zoo sib xws siv lub kaum sab xis sab SAS zoo sib xws postulate?
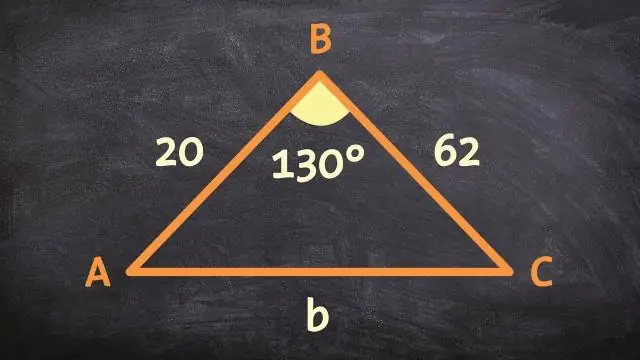
SAS Similarity Theorem hais tias yog ob sab hauv ib daim duab peb sab yog proportional rau ob sab hauv lwm daim duab peb sab thiab lub kaum sab xis nyob rau hauv ob qho tib si congruent, ces ob daim duab peb sab zoo ib yam. Ib qho kev hloov pauv zoo sib xws yog ib lossis ntau qhov kev hloov pauv tsis tu ncua ua raws li kev dilation
Puas yog cov ntsiab lus uas muaj cov tshuaj lom neeg zoo sib xws yuav pom nyob rau tib lub sijhawm lossis hauv tib pab pawg piav qhia koj cov lus teb?

Qhov no yog vim hais tias cov tshuaj muaj zog nyob ntawm tsis muaj valence electrons. Raws li nyob rau hauv ib pawg tag nrho cov ntsiab lus muaj tib yam tsis muaj valence electron uas yog vim li cas lawv muaj cov tshuaj zoo sib xws tab sis nyob rau hauv ib lub sij hawm tsis muaj valence electron sib txawv uas yog vim li cas lawv txawv nyob rau hauv chemical zog
Puas yog tag nrho cov ionic compounds muaj cov qauv lattice?

Ib qho ionic compound yog cov qauv loj heev ntawm cov ions. Cov ions muaj qhov tsis tu ncua, rov ua dua tshiab hu ua ionic lattice. Qhov no yog vim li cas cov khoom ioniccompounds tsim cov muaju nrog cov duab tsis tu ncua
