
Video: Dab tsi ntawm daim ntawv cog lus yog neon?

2024 Tus sau: Miles Stephen | [email protected]. Kawg hloov kho: 2023-12-15 23:36
A neon atom (Ne) collides nrog ib tug oxygen atom ntawm ib tug molecule (O2) nrog nws daim ntawv cog lus kev taw qhia (Daim duab E2. 8). Lub zog kinetic ntawm Ne atom yog K1= 6 × 10–21 J. Oxygen daim ntawv cog lus rigidity coefficient β yog 1.18 × 103 N/m.
Ib sab ntawm no, puas yog Neon ionic lossis covalent?
Qhov ruaj khov heev zoo gasses , suav nrog helium, neon, argon, krypton, xenon thiab radon, yog tag nrho cov ntsiab lus uas tsis yog hlau. Cov ntsiab lus no tsim kev sib raug zoo nrog ib leeg los ntawm kev sib koom electrons los ua cov sib txuas.
Ib yam li ntawd, Neon tuaj yeem tsim tau pes tsawg daim ntawv cog lus? A daim ntawv cog lus qhov twg ob atoms sib koom electrons hu ua covalent daim ntawv cog lus . Cov ntsiab lus hauv kab thib ob, lithium los ntawm fluorine, thiab daim ntawv cog lus yog li ntawd lawv ua tau ua kom ruaj khov, puv-plhaub electron configuration ntawm neon nrog 8 electrons.
Dhau li ntawm no, dab tsi Bonds muaj neon?
Txij li thaum neon yog ib qho roj av zoo, nws muaj nws tag nrho ntawm valence electrons , uas ua rau nws tsis zoo rau kev sib raug zoo nrog lwm cov atoms. Nyob rau hauv tej yam kev mob lwm yam noble gases, tshwj xeeb tshaj yog xenon thiab krypton, tuaj yeem tsim cov tebchaw hauv qhov kub thiab txias. Neon muaj nyob hauv ib qho atoms hauv nws daim ntawv elemental.
Dab tsi yog cov ntsiab lus neon react nrog?
Ib yam li txhua tus cov pa roj , nws yog heev non-reactive. Ntau npaum li ntawd, hais tias nws tsis tsim compounds nrog dab tsi. Ib yam li helium (Nws) thiab argon ( Ar ), neon floats ib ncig ntawm nws tus kheej. Nws yog non-reactive vim nws lub plhaub puv.
Pom zoo:
Dab tsi ntawm daim ntawv cog lus yog aluminium thiab oxygen?

Hauv zaj lus qhia no, peb tau kawm tias aluminium oxide yog ib qho ionic compound tsim ntawm txhuas hlau thiab oxygen. Ionic compounds tshwm sim ntawm cov hlau thiab cov hlau tsis muaj hlau thiab koom nrog kev sib pauv ntawm electrons ntawm ob lub atoms
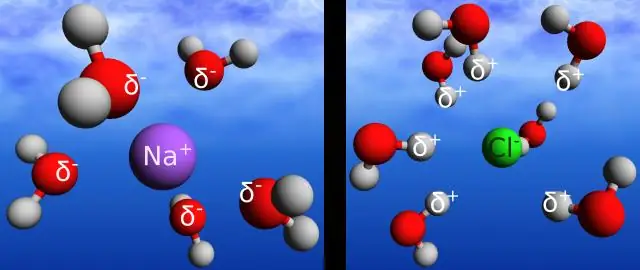
Yuav ua li cas yog daim ntawv cog lus sib txawv ntawm daim ntawv cog lus ionic?
Qhov sib txawv ntawm cov ionic thiab covalent daim ntawv cog lus yog tias covalent daim ntawv cog lus yog tsim thaum ob lub atoms sib koom electrons. Ionic bonds yog lub zog uas tuav ua ke electrostatic zog ntawm kev nyiam ntawm cov ions oppositely them. Ionic bonds muaj qhov sib txawv electronegativity ntau dua lossis sib npaug li 2
Dab tsi yog qhov txawv ntawm daim ntawv cog lus lub zog thiab daim ntawv cog lus dissociation zog?
Qhov sib txawv tseem ceeb ntawm kev sib txuas ntawm lub zog thiab lub zog bonddissociation yog tias lub zog sib txuas yog qhov nruab nrab ntawm lub zog xav tau los rhuav tshem tag nrho cov kev sib txuas ntawm tib ob hom atoms nyob rau hauv ib qho chaw sib txuas whereas daim ntawv cog lus dissociation zog yog tus nqi ntawm cov hluav taws xob xav tau los rhuav tshem ib qho kev sib koom ua ke inhomolysis
Puas yog daim ntawv cog lus hydrogen zoo ib yam li daim ntawv cog lus?
Hydrogen daim ntawv cog lus yog lub npe muab rau electrostatic kev sib cuam tshuam ntawm tus nqi zoo ntawm hydrogen atom thiab tus nqi tsis zoo ntawm oxygen atom ntawm ib qho chaw nyob sib ze. Covalent daim ntawv cog lus yog qhov sib cuam tshuam electrostatic ntawm ob lub atom hauv tib lub molecule
Dab tsi ntawm daim ntawv cog lus yog arsenic pentafluoride?

Chemical Structure Description Lub Arsenic pentafluoride molecule muaj tag nrho ntawm 5 daim ntawv cog lus Muaj 5 daim ntawv cog lus uas tsis yog H. Daim duab 2D tshuaj lom neeg cov duab ntawm Arsenic pentafluoride tseem hu ua skeletal formula, uas yog tus qauv sau rau cov organic molecules
