
Video: Muaj pes tsawg electrons nyob hauv lub plhaub sab nraud ntawm Pawg 6 cov ntsiab lus?

2024 Tus sau: Miles Stephen | [email protected]. Kawg hloov kho: 2023-12-15 23:36
Atoms ntawm pawg 1 cov ntsiab lus muaj ib qho hluav taws xob hauv lawv lub plhaub sab nrauv, thiab cov atom ntawm pawg 2 cov ntsiab lus muaj ob tug electrons nyob rau hauv lawv lub plhaub sab nraud. Qee lub ntsiab lus hauv pawg 6 thiab 7, thiab tag nrho hauv pawg 0 (tseem hu ua pawg 8) yog cov hlau tsis muaj hlau.
Ua li no, muaj pes tsawg tus electrons nyob hauv lub plhaub sab nrauv?
8 electron
Tom qab ntawd, lo lus nug yog, dab tsi yog lub npe ntawm Pawg 6 cov ntsiab lus? Pawg 6 lub ntsiab. Pawg 6, suav los ntawm IUPAC style, yog ib pab pawg ntawm cov ntsiab lus hauv lub sijhawm. Nws cov tswv cuab yog chromium (Cr), molybdenum (Mo), tungsten (W), thiab seaborgium (Sg). Cov no yog tag nrho kev hloov hlau thiab chromium , molybdenum thiab tungsten yog cov hlau refractory.
Ib sab ntawm no, pes tsawg valence electrons puas muaj pawg 6?
Pawg 5 atoms muaj 5 valence electrons . Pawg 6 atoms muaj 6 valence electrons. Pawg 7 atoms muaj 7 valence electrons.
Tus nqi ntawm Pawg 6 yog dab tsi?
Piv txwv ntawm cov nqi ion thiab pab pawg
| Pab pawg | Element | Ion nqi |
|---|---|---|
| 1 | Na | + |
| 2 | Mg | 2+ |
| 6 | O | 2- |
| 7 | Cl | - |
Pom zoo:
Lub organelle twg ua haujlwm raws li qhov chaw xa ntawv ntawm lub xov tooj ntawm tes txheeb cov proteins thiab xa lawv mus rau lawv qhov chaw xav tau sab hauv lossis sab nraud ntawm tes?
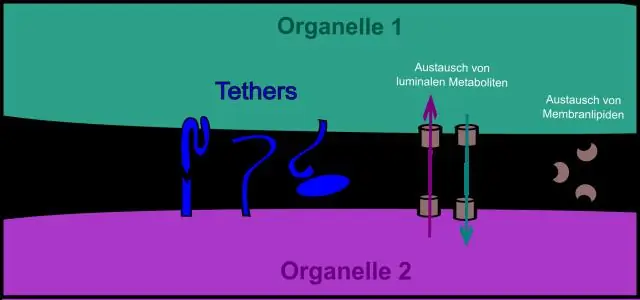
Golgi Hais txog qhov no, dab tsi organelle yog lub luag haujlwm rau kev thauj mus los? endoplasmic reticulum (ER Qhov thib ob, cov proteins txav mus los ntawm lub cell li cas? Cov proteins txav mus los endomembrane system thiab raug xa tawm los ntawm lub ntsej muag trans ntawm Golgi apparatus nyob rau hauv thauj vesicles uas txav mus los cytoplasm thiab tom qab ntawd fuse nrog plasma membrane tso tawm protein ntau mus rau sab nraum lub cell .
Vim li cas nws thiaj li hais tias lub Orthocenter ntawm daim duab peb sab obtuse yuav tsum nyob rau sab nraud ntawm daim duab peb sab?

Nws hloov tawm tias tag nrho peb qhov siab ib txwm sib tshuam ntawm tib lub ntsiab lus - lub npe hu ua orthocenter ntawm daim duab peb sab. Lub orthocenter tsis yog ib txwm nyob hauv daim duab peb sab. Yog tias daim duab peb sab yog obtuse, nws yuav nyob sab nraud. Txhawm rau ua kom qhov no tshwm sim cov kab qhov siab yuav tsum tau txuas ntxiv kom lawv hla
Muaj pes tsawg lub tangents hauv lub voj voog uas sib tshuam hauv ob lub ntsiab lus muaj?

Thaum ib lub voj voog nyob tag nrho hauv ib leeg yam tsis tau kov, tsis muaj tangent. Thaum ob lub voj voog kov ib leeg sab hauv 1 cov tangent tuaj yeem kos rau lub voj voog. Thaum ob lub voj voog sib tshuam hauv ob lub ntsiab lus tiag tiag thiab sib txawv, 2 cov tangents tuaj yeem kos rau lub voj voog
Puas yog cov ntsiab lus uas muaj cov tshuaj lom neeg zoo sib xws yuav pom nyob rau tib lub sijhawm lossis hauv tib pab pawg piav qhia koj cov lus teb?

Qhov no yog vim hais tias cov tshuaj muaj zog nyob ntawm tsis muaj valence electrons. Raws li nyob rau hauv ib pawg tag nrho cov ntsiab lus muaj tib yam tsis muaj valence electron uas yog vim li cas lawv muaj cov tshuaj zoo sib xws tab sis nyob rau hauv ib lub sij hawm tsis muaj valence electron sib txawv uas yog vim li cas lawv txawv nyob rau hauv chemical zog
Lub sij hawm twg yog siv rau electrons hauv lub plhaub sab nraud?

Kev piav qhia: Lub plhaub sab nraud yog hu ua 'valence plhaub'. Yog li ntawd, cov electrons nyob rau hauv lub plhaub sab nraud yog hu ua 'valence electrons'
