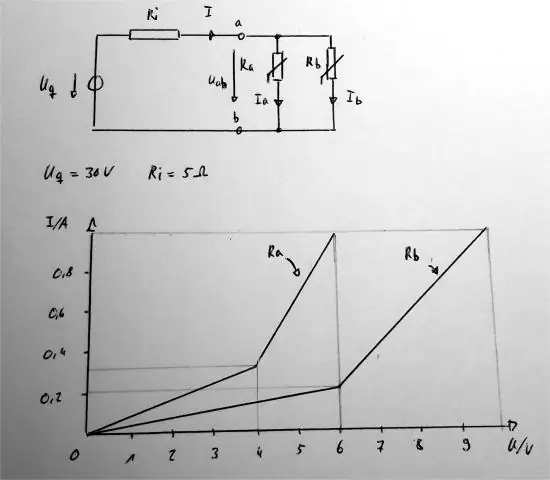
Cov txheej txheem:
Video: Yuav ua li cas koj txiav txim lub freezing point?

2024 Tus sau: Miles Stephen | [email protected]. Kawg hloov kho: 2023-12-15 23:36
Tswv yim:
- Kauj ruam 1: xam tus qhov chaw khov kev nyuaj siab ntawm benzene. Tf = ( Qhov chaw khov ntawm cov kuab tshuaj ntshiab) - ( Qhov chaw khov ntawm kev daws)
- Kauj ruam 2: xam lub molal concentration ntawm cov tshuaj. molality = moles ntawm solute / kg ntawm hnyav.
- Kauj Ruam 3: xam Kf ntawm qhov kev daws teeb meem. Tf = (Kf) (m)
Yog li ntawd, dab tsi yog lub mis rau khov point?
Cov qhov chaw khov kev nyuaj siab ∆T = KF·m qhov twg KF yog molal qhov chaw khov kev nyuaj siab tas li thiab m yog tus molality ntawm tus solute. Rearrangement muab: mol solute = (m) x (kg hnyav) qhov twg kg ntawm hnyav yog qhov hnyav ntawm cov kuab tshuaj (lauric acid) hauv qhov sib tov. Qhov no muab cov moles ntawm solute.
Tsis tas li ntawd, ua li cas koj thiaj paub tias cov tshuaj twg muaj qhov qis tshaj qhov khov? Teb thiab piav qhia: Qhov chaw khov ntawm ib tug ntshiab solvent nyob ntawm tus nqi ntawm solute uas tau yaj nyob rau hauv nws. Nyob rau hauv thiaj li yuav txiav txim siab seb cov tshuaj twg muaj qhov chaw txias tshaj plaws , peb yuav tsum tau saib cov molality thiab seb cov solute yog ionic los yog covalent.
Tsis tas li ntawd, koj yuav txiav txim siab qhov chaw khov ntawm cov kua li cas?
Raws li nrog khov , lub melting point kub nyob twj ywm ntawm tib nyeem ntawv kom txog rau thaum cov khoom ua ua kua . Ntxig tus pas ntsuas kub rau hauv slush, ua ntej koj nyob nraum ntsuas tig kiag li ua kua . Cia tus pas ntsuas kub nyob rau ntawd kom txog rau thaum lub taw tes thaum nws ua tag nrho ua kua . Sau cov kub thaum ntawd tshwm sim.
Puas yog qhov chaw khov kho kev nyuaj siab zoo lossis tsis zoo?
ΔTtrs, kev hloov pauv hauv theem hloov taw tes , uas yog ib txwm tsis zoo rau freezing point kev nyuaj siab thiab zoo rau boil taw tes qhov siab.
Pom zoo:
Yuav ua li cas koj txiav txim siab oxidation lub xeev ntawm carbon nyob rau hauv cov organic tebchaw?

Txhawm rau xam lub xeev oxidation rau carbon, siv cov lus qhia hauv qab no: Hauv C-H daim ntawv cog lus, H raug kho zoo li nws muaj oxidation xeev ntawm +1. Rau cov pa roj carbon monoxide ntau dua electronegative tsis-hlau X, xws li nitrogen, oxygen, sulfur los yog halogens, txhua C-X daim ntawv cog lus yuav ua rau kom lub xeev oxidation ntawm cov pa roj carbon ntau dua 1
Yuav ua li cas koj txiav txim siab lub active site ntawm ib tug enzyme?
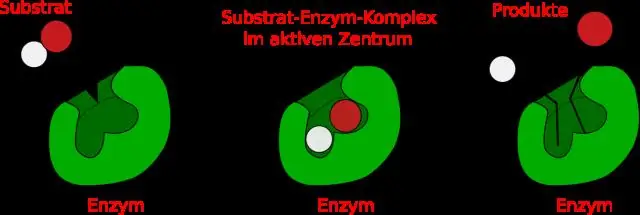
Kev Taw Qhia. Cov chaw nquag yog cov cheeb tsam feem ntau nyob rau saum npoo ntawm cov enzymes tshwj xeeb los ntawm cov xwm txheej thaum evolution uas yog catalyze cov tshuaj tiv thaiv los yog lub luag hauj lwm rau substrate khi. Qhov chaw nquag tuaj yeem yog, yog li ntawd, muab faib ua ob ntu, uas suav nrog qhov chaw catalytic thiab substrate binding site (1)
Yuav ua li cas koj txiav txim siab npaum li cas ntawm cov kua qaub uas yuav tsum tau neutralize lub hauv paus?

Kev daws qhov teeb meem Acid-Base Neutralization Kauj Ruam 1: Muab xam cov moles ntawm OH-. Molarity = moles / ntim. moles = Molarity x Volume. moles OH- = 0.02 M/100 milliliters. Kauj ruam 2: Xam qhov ntim ntawm HCl xav tau. Molarity = moles / ntim. Volume = moles/molarity. Volume = moles H+/0.075 Molarity
Yuav ua li cas koj txiav txim siab yog tias daim ntawv cog lus yog polar tsis muaj lub rooj electronegativity?

Kauj Ruam 2: Txheeb xyuas txhua daim ntawv cog lus raws li polar lossis nonpolar. (Yog hais tias qhov sib txawv ntawm electronegativity rau cov atoms nyob rau hauv ib daim ntawv cog lus yog ntau tshaj 0.4, peb xav txog daim ntawv cog lus polar. Yog hais tias qhov sib txawv ntawm electronegativity tsawg dua 0.4, daim ntawv cog lus yog qhov tseem ceeb nonpolar.) Yog tias tsis muaj polar bonds, lub molecule yog tsis muaj polar
Yuav ua li cas koj txiav txim siab uas yog tus nqi txiav txim kauj ruam?

Tus nqi txiav txim siab kauj ruam yog kauj ruam qeeb tshaj plaws ntawm cov tshuaj tiv thaiv tshuaj uas txiav txim siab qhov nrawm (tus nqi) uas tag nrho cov tshuaj tiv thaiv ua tiav. Teb Tus nqi txiav txim kauj ruam yog kauj ruam thib ob vim nws yog kauj ruam qeeb. 2NO + 2H2 → N2 + 2H2O. Cov intermediates hauv cov tshuaj tiv thaiv no yog N2O2 thiab N2O
