Cov txheej txheem:
- Ua raws li cov cai no kom sib npaug qhov sib npaug redox yooj yim:
- Feem ntau, txhawm rau sib npaug ntawm qhov sib npaug, ntawm no yog yam peb yuav tsum ua:
Video: Koj ua li cas sib npaug tshuaj sib npaug nrog cov lej oxidation?

2024 Tus sau: Miles Stephen | [email protected]. Kawg hloov kho: 2023-12-15 23:36
Hauv oxidation naj npawb txoj kev, koj txiav txim qhov oxidation naj npawb ntawm tag nrho cov atoms. Tom qab ntawd koj muab cov atoms uas tau hloov los ntawm me me tus lej . Koj tab tom ua rau tag nrho cov poob ntawm electrons sib npaug rau tag nrho qhov nce ntawm electrons. Ces koj tshuav nyiaj li cas lwm cov atoms.
Raws li, koj ua li cas sib npaug oxidation txo qhov sib npaug?
Ua raws li cov cai no kom sib npaug qhov sib npaug redox yooj yim:
- Sau cov oxidation thiab txo ib nrab-reactions rau cov tsiaj uas txo los yog oxidized.
- Muab cov kev sib piv ib nrab los ntawm tus lej tsim nyog kom lawv muaj cov lej sib npaug ntawm cov hluav taws xob.
- Ntxiv ob qhov sib npaug kom tshem tawm cov electrons.
Qhov thib ob, koj ua li cas sib npaug sib npaug? Txoj Kev 1 Ua ib qho piv txwv
- Sau koj qhov kev sib npaug.
- Sau tus naj npawb ntawm atoms rau ib lub caij.
- Txuag hydrogen thiab oxygen rau qhov kawg, vim lawv feem ntau ntawm ob sab.
- Pib nrog ib lub ntsiab lus.
- Siv cov coefficient kom sib npaug ntawm ib qho carbon atom.
- Ntsuas cov hydrogen atoms tom ntej.
- Ntsuas cov pa oxygen atoms.
Tsis tas li, koj ua li cas sib npaug tshuaj sib npaug yooj yim?
Feem ntau, txhawm rau sib npaug ntawm qhov sib npaug, ntawm no yog yam peb yuav tsum ua:
- Suav cov atoms ntawm txhua lub caij hauv cov reactants thiab cov khoom.
- Siv cov coefficients; muab lawv tso rau pem hauv ntej ntawm lub tebchaw raws li xav tau.
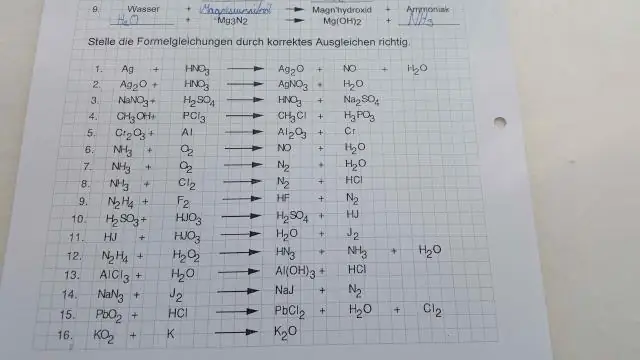
Puas yog C o2 co2 cov tshuaj tiv thaiv redox?
Yog C + O2 = CO2 ib qho intramolecular tshuaj tiv thaiv redox los tsis? Ib qho reactant (carbon) yog oxidized thiab lwm tus (oxygen) txo. Yog li qhov no yog qhov yooj yim intermolecular tshuaj tiv thaiv redox . Ib qho tshuaj tiv thaiv nyob rau hauv uas ib los yog ntau tshaj reactants / cov khoom tshwm sim los ua cov ntsiab lus ntshiab yuav tsum yog a tshuaj tiv thaiv redox.
Pom zoo:
Yuav daws qhov tsis sib npaug ntawm cov kab sib npaug thiab cov kab sib npaug sib npaug li cas?

Kev daws qhov tsis sib npaug ntawm qhov sib npaug yog zoo ib yam li kev daws cov kab sib npaug. Qhov sib txawv tseem ceeb yog koj tig lub cim tsis sib xws thaum faib lossis muab faib los ntawm tus lej tsis zoo. Graphing linear inequalities muaj ob peb qhov sib txawv ntxiv. Qhov uas yog qhov ntxoov ntxoo muaj xws li qhov tseem ceeb uas qhov kev tsis sib xws ntawm cov kab tawm muaj tseeb
Yuav ua li cas koj thiaj nrhiav tau cov lej ntawm cov lej lej lossis cov lej geometric?
Cov mis rau qhov suav ntawm n cov ntsiab lus ntawm geometric ib ntus yog muab los ntawm Sn = a[(r^n - 1)/(r - 1)], qhov twg a yog thawj lub sij hawm, n yog lub sij hawm tus lej thiab r yog tus qhov piv txwv
Cov coefficients hauv kev sib npaug tshuaj sib npaug qhia koj li cas txog cov reactants thiab cov khoom?

Cov coefficients ntawm kev sib npaug chemicalequation qhia peb cov txheeb ze ntawm moles ntawm reactants thiab cov khoom. Hauv kev daws teeb meem stoichiometric, kev hloov pauv ntawm moles ntawm reactants rau moles ntawm cov khoom raug siv. Hauv kev suav loj, cov molar loj yog xav tau los hloov cov huab hwm coj mus rau moles
Dab tsi yog cov lej suav tag nrho cov lej suav lej thiab lej lej?
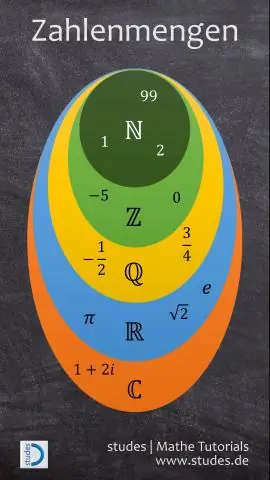
Cov lej tiag yog feem ntau muab faib ua cov lej tsis sib haum xeeb thiab tsis sib haum xeeb. Cov zauv rational suav nrog tag nrho cov lej thiab feem. Txhua tus lej tsis zoo thiab tag nrho cov lej ua rau cov lej suav. Tag nrho cov lej suav nrog txhua tus lej ntuj thiab xoom
Dab tsi yog qhov sib txawv ntawm cov kab lus sib npaug thiab qhov sib npaug sib npaug?
Cov kab lus sib npaug muaj qhov muaj txiaj ntsig zoo ib yam tab sis tau nthuav tawm nyob rau hauv ib hom ntawv sib txawv uas siv cov khoom ntawm cov lej xws li ax + bx = (a + b) x yog cov kab lus sib npaug. nruj me ntsis, lawv tsis yog 'sib npaug', yog li peb yuav tsum siv 3 kab sib luag hauv 'sib npaug' es tsis yog 2 raws li qhia ntawm no
