Cov txheej txheem:

Video: Dab tsi ntawm daim ntawv cog lus muaj nyob hauv graphite?

2024 Tus sau: Miles Stephen | [email protected]. Kawg hloov kho: 2023-12-15 23:36
Graphite muaj cov qauv loj heev uas:
- txhua cov pa roj carbon atom koom nrog peb lwm cov pa roj carbon atom los ntawm covalent bonds .
- cov pa roj carbon atoms tsim cov khaubncaws sab nraud povtseg nrog hexagonal kev npaj ntawm atoms.
- cov txheej muaj zog tsis muaj zog ntawm lawv.
- Txhua cov pa roj carbon atom muaj ib qho uas tsis yog-bonded sab nraud electron, uas dhau los ua delocalised.
Ntawm no, vim li cas graphite tsuas muaj 3 daim ntawv cog lus?
Cov orbitals no yuav sib tshooj nrog ib leeg, yog li txhua tus carbon daim ntawv 3 daim ntawv cog lus nrog rau lwm cov pa roj carbon daim ntawv ib txheej hexagonal. Cov carbons tsim peb daim ntawv cog lus xwb vim sp 2 hybridized (li no -ene suffix).
Tom qab ntawd, lo lus nug yog, hom kev sib txuas yog dab tsi hauv pob zeb diamond? covalent bonds
Kuj Paub, dab tsi yog cov khoom ntawm pob zeb diamond thiab graphite?
Covalent Network Solids yog cov khoom loj heev zoo li pob zeb diamond , graphite thiab silicon dioxide (silicon (IV) oxide).
Lub cev muaj zog ntawm pob zeb diamond
- Nws muaj qhov siab melting point (yuav luag 4000 ° C).
- nyuaj heev.
- tsis ua hluav taws xob.
- yog insoluble nyob rau hauv dej thiab organic solvents.
Hom qauv twg yog pob zeb diamond thiab graphite daim ntawv?
Pob zeb diamond thiab graphite txawv daim ntawv ntawm cov khoom carbon. Lawv ob leeg suav nrog covalent network loj heev cov qauv ntawm carbon atoms, koom ua ke los ntawm covalent bonds.
Pom zoo:
Yuav ua li cas yog daim ntawv cog lus sib txawv ntawm daim ntawv cog lus ionic?
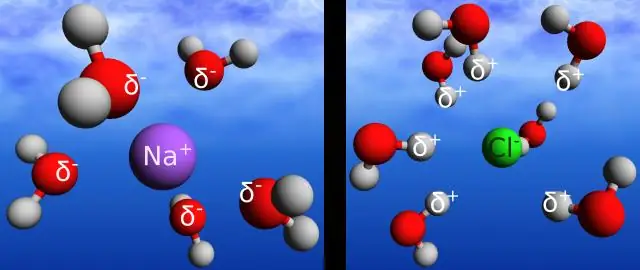
Qhov sib txawv ntawm cov ionic thiab covalent daim ntawv cog lus yog tias covalent daim ntawv cog lus yog tsim thaum ob lub atoms sib koom electrons. Ionic bonds yog lub zog uas tuav ua ke electrostatic zog ntawm kev nyiam ntawm cov ions oppositely them. Ionic bonds muaj qhov sib txawv electronegativity ntau dua lossis sib npaug li 2
Dab tsi yog qhov txawv ntawm daim ntawv cog lus lub zog thiab daim ntawv cog lus dissociation zog?
Qhov sib txawv tseem ceeb ntawm kev sib txuas ntawm lub zog thiab lub zog bonddissociation yog tias lub zog sib txuas yog qhov nruab nrab ntawm lub zog xav tau los rhuav tshem tag nrho cov kev sib txuas ntawm tib ob hom atoms nyob rau hauv ib qho chaw sib txuas whereas daim ntawv cog lus dissociation zog yog tus nqi ntawm cov hluav taws xob xav tau los rhuav tshem ib qho kev sib koom ua ke inhomolysis
Puas yog daim ntawv cog lus hydrogen zoo ib yam li daim ntawv cog lus?
Hydrogen daim ntawv cog lus yog lub npe muab rau electrostatic kev sib cuam tshuam ntawm tus nqi zoo ntawm hydrogen atom thiab tus nqi tsis zoo ntawm oxygen atom ntawm ib qho chaw nyob sib ze. Covalent daim ntawv cog lus yog qhov sib cuam tshuam electrostatic ntawm ob lub atom hauv tib lub molecule
Cov ntawv cog lus dab tsi muaj nyob hauv ib qho piv txwv ntawm h2o S?

Hauv H2O molecule, ob lub dej molecules yog sib koom ua ke los ntawm Hydrogen daim ntawv cog lus tab sis qhov kev sib cog lus ntawm ob H - O daim ntawv cog lus hauv cov dej molecule yog covalent
Dab tsi hydrocarbon muaj ob daim ntawv cog lus hauv nws cov pob txha carbon?

Organic compounds uas muaj carbon-carbon ob daim ntawv cog lus hu ua alkenes. Cov pa roj carbon atoms koom nrog ob daim ntawv cog lus yog sp2 hybridized. Ob qho yooj yim alkenes yog ethene (C2H4) thiab propene (C3H6). Alkenes nyob rau hauv uas txoj hauj lwm ntawm ob daim ntawv cog lus sib txawv yog txawv molecules
