
Video: Plaub tus lej quantum yog dab tsi rau electrons thiab lawv txhais li cas?

2024 Tus sau: Miles Stephen | [email protected]. Kawg hloov kho: 2023-12-15 23:36
Cov plaub tus lej quantum siv los piav txog qhov electrons n = 2, ℓ = 1, m = 1, 0, lossis -1, thiab s = 1/2 (the electrons muaj parallel spin).
Tsis tas li ntawd, plaub tus lej quantum piav qhia txog dab tsi ntawm ib qho hluav taws xob?
Ua kom tiav piav ib hluav taws xob hauv atom, plaub tus lej quantum xav tau: zog (n), angular momentum (ℓ), magnetic moment (mℓ), thiab spin (ms). Thawj quantum tus lej piav tus hluav taws xob plhaub, lossis qib zog, ntawm ib qho atom.
Ib yam li ntawd, koj ua li cas thiaj nrhiav tau tus lej quantum ntawm electrons? Yuav Ua Li Cas Txiav Txim Electrons Nrog Quantum Numbers
- suav cov Orbitals tag nrho.
- Ntxiv cov Electrons rau Txhua Lub Orbital Tag Nrho.
- Txheeb xyuas Subshell Qhia los ntawm Angular Quantum Number.
- Ntxiv cov Electrons los ntawm Tag Nrho Subshells.
- Ntxiv cov Electrons los ntawm Tag Nrho Subshells rau Cov Los Ntawm Tag Nrho Orbitals.
- Nrhiav cov Vales raug cai rau Magnetic Quantum Number.
Kuj kom paub, plaub tus lej quantum yog dab tsi?
Hauv atoms, muaj tag nrho ntawm plaub tus lej quantum: tus thawj quantum tooj (n), lub orbital angular momentum quantum tooj (l), ua magnetic quantum tooj (ml), thiab electron spin quantum tooj (ms).
Subshell yog dab tsi?
A subshell yog ib tug subdivision ntawm electron plhaub sib cais los ntawm electron orbitals. Subshells yog sau npe s, p, d, thiab f hauv kev teeb tsa hluav taws xob.
Pom zoo:
Dab tsi yog tus lej ntuj thiab tus lej suav nrog piv txwv?
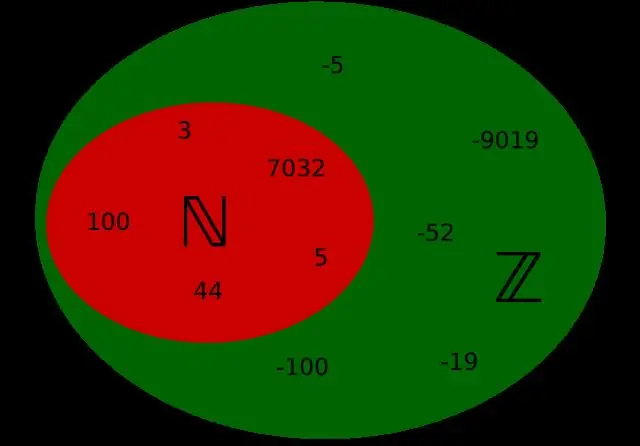
Cov lej ntuj yog tag nrho cov lej 1, 2, 3, 4… Lawv yog cov lej koj feem ntau suav thiab lawv yuav txuas ntxiv mus rau qhov tsis muaj tseeb. Tag nrho cov lej yog txhua tus lej suav nrog 0 piv txwv. 0, 1, 2, 3, 4… Cov zauv suav nrog tag nrho cov lej thiab lawv qhov tsis zoo sib piv xws li.
Dab tsi yog cov lej suav tag nrho cov lej suav lej thiab lej lej?
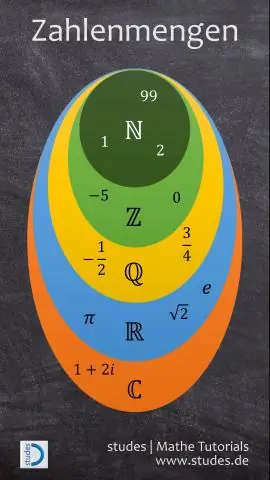
Cov lej tiag yog feem ntau muab faib ua cov lej tsis sib haum xeeb thiab tsis sib haum xeeb. Cov zauv rational suav nrog tag nrho cov lej thiab feem. Txhua tus lej tsis zoo thiab tag nrho cov lej ua rau cov lej suav. Tag nrho cov lej suav nrog txhua tus lej ntuj thiab xoom
Dab tsi yog tus lej loj thiab tus lej atomic?

Tus lej loj (xws li tsab ntawv A) txhais tau tias yog tag nrho cov protons thiab neutrons hauv ib lub atom. Xav txog cov lus hauv qab no, uas qhia cov ntaub ntawv los ntawm thawj rau lub ntsiab lus ntawm lub sijhawm. Xav txog lub ntsiab helium. Nws tus lej atomic yog 2, yog li nws muaj ob lub protons hauv nws cov nucleus
Dab tsi yog tus lej thiab tus lej tiag?
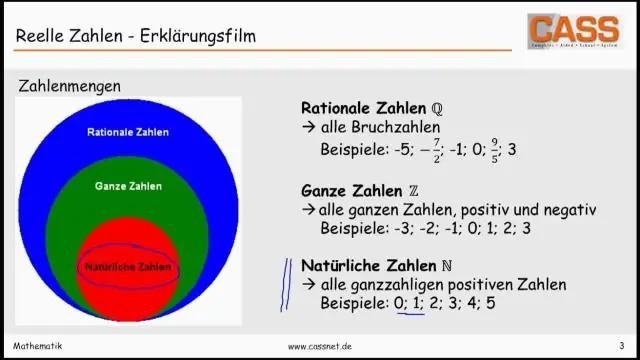
Cov hom tseem ceeb): Cov lej suav {1, 2, 3,} feem ntau hu ua tus lej ntuj; Txawm li cas los xij, lwm cov ntsiab lus suav nrog 0, yog li cov lej tsis zoo {0, 1, 2, 3,} kuj hu ua tus lej ntuj. Cov lej ntuj suav nrog 0 tseem hu ua tus lej tag nrho.): Cov lej tiag tiag uas tsis yog qhov tseeb
Qhov twg ntawm cov plaub hau plaub hau yog ib txwm plaub hau plaub hau?
square Tsis tas li ntawd nug, qhov ntsuas ntawm ib lub quadrilateral tsis tu ncua yog dab tsi? Yog, sab hauv lub kaum sab xis ntawm txhua lub ces kaum ntawm ib lub quadrilateral tsis tu ncua yog txhua 90 degrees (360 degrees / 4 fab).
