
Video: Dab tsi yog daim ntawv cog lus qis tshaj plaws hauv biology?

2024 Tus sau: Miles Stephen | [email protected]. Kawg hloov kho: 2023-12-15 23:36
Daim duab ntawm intramolecular polar covalent kev sib raug zoo nyob rau hauv H20 molecules thiab hydrogen kev sib raug zoo ntawm O thiab H atoms. London dispersion rog, nyob rau hauv qeb ntawm van der Waal rog: Cov no yog cov tsis muaj zog Lub zog ntawm lub cev muaj zog thiab muaj nyob nruab nrab ntawm txhua hom molecules, txawm tias ionic lossis covalent-polar lossis nonpolar.
Ib yam li ntawd, qhov twg yog daim ntawv cog lus qis tshaj?
Cov ionic daim ntawv cog lus feem ntau yog cov tsis muaj zog ntawm qhov tseeb tshuaj daim ntawv cog lus uas khi atoms rau atoms.
Ib yam li ntawd, qhov kev sib raug zoo tshaj plaws hauv biology yog dab tsi? Cov ruaj khov uas muaj nyob rau hauv biochemicals yog covalent daim ntawv cog lus , zoo li cov daim ntawv cog lus uas tuav cov atoms ua ke nyob rau hauv ib tug neeg lub hauv paus qhia nyob rau hauv daim duab 1.3. Ib covalent daim ntawv cog lus yog tsim los ntawm kev sib koom ntawm ib khub ntawm electrons ntawm cov atoms uas nyob ib sab.
Hais txog qhov no, Van der Waals puas yog daim ntawv cog lus qis tshaj plaws?
Kuv hais tias hydrogen daim ntawv cog lus muaj zog heev, tab sis qhov ntawd tsuas yog piv rau lwm tus van der Waals ' zog. Piv rau hais, ib tug covalent daim ntawv cog lus , ib hydrogen daim ntawv cog lus yog kwv yees li ib feem kaum ntawm lub zog ntawd. Lub dipole-dipole daim ntawv cog lus tseem tsis muaj zog, thiab cov dispersion rog yog cov tsis muaj zog ntawm Van De Waals ' zog.
Koj ua li cas thiaj paub tias daim ntawv cog lus tsis muaj zog?
Yog li peb ua tau txiav txim muaj zog los yog qaug zog covalent daim ntawv cog lus.
- Bond zog nce nrog ntau qhov sib npaug ntawm cov ntawv cog lus hauv atom vim nws nyuaj rau kev rhuav tshem cov ntawv cog lus.
- Kev pom ntawm ib leeg khub ua rau kev sib raug zoo tsis muaj zog.
- Bonds tsim los ntawm hybridisation muaj zog tshaj cov ntshiab atomic bonds.
- Polar bonds muaj zog dua li cov ntawv cog lus yooj yim.
Pom zoo:
Yuav ua li cas yog daim ntawv cog lus sib txawv ntawm daim ntawv cog lus ionic?
Qhov sib txawv ntawm cov ionic thiab covalent daim ntawv cog lus yog tias covalent daim ntawv cog lus yog tsim thaum ob lub atoms sib koom electrons. Ionic bonds yog lub zog uas tuav ua ke electrostatic zog ntawm kev nyiam ntawm cov ions oppositely them. Ionic bonds muaj qhov sib txawv electronegativity ntau dua lossis sib npaug li 2
Dab tsi yog qhov txawv ntawm daim ntawv cog lus lub zog thiab daim ntawv cog lus dissociation zog?
Qhov sib txawv tseem ceeb ntawm kev sib txuas ntawm lub zog thiab lub zog bonddissociation yog tias lub zog sib txuas yog qhov nruab nrab ntawm lub zog xav tau los rhuav tshem tag nrho cov kev sib txuas ntawm tib ob hom atoms nyob rau hauv ib qho chaw sib txuas whereas daim ntawv cog lus dissociation zog yog tus nqi ntawm cov hluav taws xob xav tau los rhuav tshem ib qho kev sib koom ua ke inhomolysis
Puas yog daim ntawv cog lus hydrogen zoo ib yam li daim ntawv cog lus?
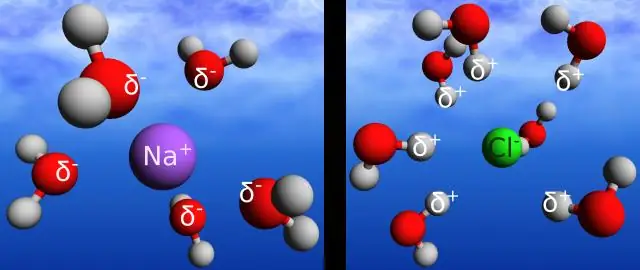
Hydrogen daim ntawv cog lus yog lub npe muab rau electrostatic kev sib cuam tshuam ntawm tus nqi zoo ntawm hydrogen atom thiab tus nqi tsis zoo ntawm oxygen atom ntawm ib qho chaw nyob sib ze. Covalent daim ntawv cog lus yog qhov sib cuam tshuam electrostatic ntawm ob lub atom hauv tib lub molecule
Qhov tsawg tshaj plaws units ntawm ib daim ntawv cog lus ionic yog dab tsi?

Teb thiab piav qhia: Chav tsev me tshaj plaws ntawm daim ntawv cog lus ionic yog cov qauv qauv, uas yog qhov tsawg tshaj plaws ntawm cov atoms hauv cov qauv siv lead ua ionic
Daim ntawv yooj yim tshaj plaws ntawm 10 tshaj 12 yog dab tsi?

Simplify 10/12 rau daim ntawv yooj yim tshaj plaws. Online simplify fractions lub laij lej kom txo tau 10/12 rau cov nqe lus qis tshaj sai thiab yooj yim. 10/12 Cov Lus Teb Yooj Yim: 10/12 = 5/6
