
Video: Puas tsim cov ntawv cog lus tso tawm lub zog?
2024 Tus sau: Miles Stephen | [email protected]. Kawg hloov kho: 2023-12-15 23:36
Hauv txhua hom tshuaj tiv thaiv, daim ntawv cog lus yog tawg thiab reassembled rau daim ntawv cov khoom tshiab. Txawm li cas los xij, hauv exothermic, endothermic, thiab tag nrho cov tshuaj tiv thaiv, nws yuav siv sij hawm zog txhawm rau rhuav cov tshuaj uas twb muaj lawm daim ntawv cog lus thiab zog yog tso tawm thaum nov daim ntawv cog lus.
Tsis tas li ntawd, puas yog lub zog tso tawm thaum muaj daim ntawv cog lus?
Zog yog absorbed kom tawg daim ntawv cog lus . Bond -breaking yog ib qho txheej txheem endothermic. Zog yog tso tawm thaum tshiab daim ntawv cog lus . Seb cov tshuaj tiv thaiv yog endothermic los yog exothermic nyob ntawm qhov sib txawv ntawm cov zog yuav tsum tau tawg daim ntawv cog lus thiab cov zog tso tawm thaum tshiab daim ntawv cog lus.
Ib tug kuj yuav nug, vim li cas thiaj li tsim daim ntawv cog lus exothermic? Thaum twg daim ntawv cog lus yog tsim lub cev poob zog thiab li no tsub kom nws stability (uas yog qhov kawg motive). Txij li thaum lawv yog ib tug txo nyob rau hauv lub zog, lub zog poob yog tso tawm raws li tshav kub zog thiab yog li nws yog ib tug exothermic txheej txheem.
Ntawm no, Bonds puas khaws lub zog?
Qhov tshaj zog ntawm 794 kJ / mol raug tso tawm raws li cua sov, uas peb tuaj yeem siv los ua noj peb cov zaub mov, thiab lwm yam. Yog li, tshuaj daim ntawv cog lus ua tsis yog khw ” zog . Cov zog rau kev tawg daim ntawv cog lus los tsuas yog thaum muaj zog daim ntawv cog lus yog tsim los hloov. Tab sis nws yuav siv sij hawm zog txhawm rau txhawm rau pab pawg phosphate los ntawm ATP.
Yuav ua li cas tshwm sim thaum chemical bond tsim?
A chemical bond yog ib tug ntev attraction ntawm atoms, ions los yog molecules uas enables lub tsim ntawm tshuaj tshuaj sib xyaw. Cov daim ntawv cog lus Tej zaum yuav tshwm sim los ntawm electrostatic quab yuam ntawm attraction ntawm oppositely them ions raws li nyob rau hauv ionic daim ntawv cog lus los yog los ntawm kev sib koom ntawm electrons li hauv covalent bonds.
Pom zoo:
Yuav ua li cas yog daim ntawv cog lus sib txawv ntawm daim ntawv cog lus ionic?
Qhov sib txawv ntawm cov ionic thiab covalent daim ntawv cog lus yog tias covalent daim ntawv cog lus yog tsim thaum ob lub atoms sib koom electrons. Ionic bonds yog lub zog uas tuav ua ke electrostatic zog ntawm kev nyiam ntawm cov ions oppositely them. Ionic bonds muaj qhov sib txawv electronegativity ntau dua lossis sib npaug li 2
Dab tsi yog qhov txawv ntawm daim ntawv cog lus lub zog thiab daim ntawv cog lus dissociation zog?
Qhov sib txawv tseem ceeb ntawm kev sib txuas ntawm lub zog thiab lub zog bonddissociation yog tias lub zog sib txuas yog qhov nruab nrab ntawm lub zog xav tau los rhuav tshem tag nrho cov kev sib txuas ntawm tib ob hom atoms nyob rau hauv ib qho chaw sib txuas whereas daim ntawv cog lus dissociation zog yog tus nqi ntawm cov hluav taws xob xav tau los rhuav tshem ib qho kev sib koom ua ke inhomolysis
Puas yog daim ntawv cog lus hydrogen zoo ib yam li daim ntawv cog lus?
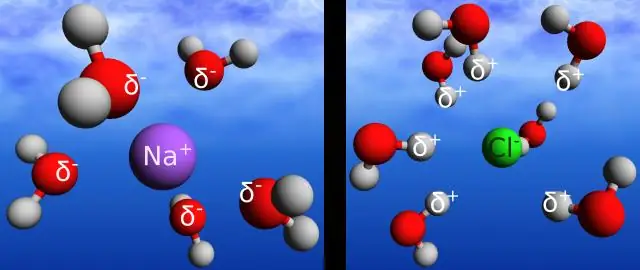
Hydrogen daim ntawv cog lus yog lub npe muab rau electrostatic kev sib cuam tshuam ntawm tus nqi zoo ntawm hydrogen atom thiab tus nqi tsis zoo ntawm oxygen atom ntawm ib qho chaw nyob sib ze. Covalent daim ntawv cog lus yog qhov sib cuam tshuam electrostatic ntawm ob lub atom hauv tib lub molecule
Txoj kev faib cov ntaub ntawv twg muab cov ntaub ntawv sib npaug ntawm cov ntaub ntawv lossis cov kev tshuaj xyuas hauv txhua chav kawm cov ntaub ntawv?

Quantile. txhua chav kawm muaj cov yam ntxwv sib npaug. Ib qho kev faib tawm ntau yog zoo haum rau cov ntaub ntawv faib tawm. Quantile muab tib tus lej ntawm cov ntaub ntawv muaj nuj nqis rau txhua chav kawm
Puas yog cua sov tso tawm thaum daim ntawv cog lus tsim?

Hauv txhua hom tshuaj tiv thaiv, daim ntawv cog lus tau tawg thiab rov ua dua los tsim cov khoom tshiab. Txawm li cas los xij, nyob rau hauv exothermic, endothermic, thiab tag nrho cov tshuaj tiv thaiv, nws yuav siv lub zog los rhuav tshem cov tshuaj uas twb muaj lawm thiab lub zog raug tso tawm thaum daim ntawv cog lus tshiab
