
Video: Acids thiab ntsev yog dab tsi?

2024 Tus sau: Miles Stephen | [email protected]. Kawg hloov kho: 2023-12-15 23:36
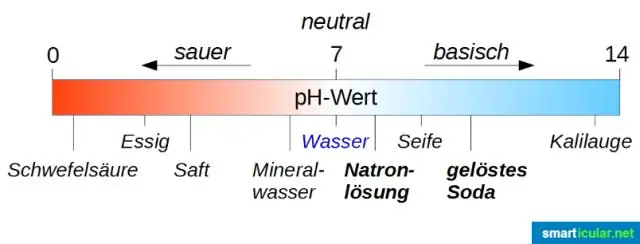
Ib kua qaub txhais tau tias yog ib yam khoom uas nws cov dej qab zib qab zib, tig xiav litmus liab thiab neutralizes hauv paus . Ntsev yog ib yam khoom nruab nrab uas nws cov kua dej tsis cuam tshuam litmus. Raws li Faraday: kua qaub , bases, thiab ntsev yog hu ua electrolytes.
Kuj yuav paub yog, dab tsi yog acid puag thiab ntsev nrog piv txwv?
Feem ntau piv txwv muaj xws li sodium hydroxide, magnesium hydroxide, sodium hydrogen carbonate (sodium bicarbonate), sodium hypochlorite thiab ammonia. Neutralization yog ib qho tshuaj tiv thaiv ntawm ib qho kua qaub thiab ib qho alkali uas ua rau a ntsev thiab dej. Cov kua qaub tuaj yeem cuam tshuam nrog qee cov hlau los ua ib qho ntsev thiab hydrogen gas.
Ib yam li ntawd, siv cov acids hauv paus thiab ntsev yog dab tsi? Hauv kev lag luam chemical, kua qaub react nyob rau hauv neutralization tshwm sim los tsim ntsev . Piv txwv li, nitric kua qaub reacts nrog ammonia los tsim ammonium nitrate, chiv. Tsis tas li ntawd, carboxylic kua qaub tuaj yeem esterified nrog cawv, tsim cov esters.
Kuj tseem paub yog, qhov sib txawv ntawm acid puag thiab ntsev yog dab tsi?
Acid muaj ib tug loj npaum li cas ntawm ions thiab qaub nyob rau hauv saj. Ntsev yog tsim thaum kua qaub thiab puag ob qho tib si sib xyaw ua ke thiab yog neutralized. Cov ions tsis zoo tsim dej thaum cov ions zoo tsim ntsev . Txawm li cas los xij, ob qho tib si kua qaub thiab hauv paus yog corrosive nyob rau hauv cov xwm, thiab kuaj kua qaub thiab hauv paus tuaj yeem ua mob rau daim tawv nqaij thiab cov hlau.
Dab tsi yog hu ua base?
Hauv chemistry, a puag yog ib hom tshuaj uas pub electrons, txais cov protons, lossis tso tawm hydroxide (OH-) ions hauv cov kua dej. Bases tso saib tej yam ntxwv yam ntxwv uas yuav siv tau los pab txheeb xyuas lawv. Hom ntawm hauv paus suav nrog Arrhenius puag , Brosted-Lowry puag , thiab Lewis puag.
Pom zoo:
Dab tsi yog lub npe ntsev tsim los ntawm neutralization ntawm hydrochloric acid thiab sodium hydroxide?
Kev piav qhia: Cov tshuaj tiv thaiv ntawm sodium hydroxide (NaOH) thiab hydrochloric acid (HCl) yog cov tshuaj tiv thaiv nruab nrab uas ua rau muaj ntsev, sodium chloride (NaCl), thiab dej (H2O). Nws yog ib qho tshuaj tiv thaiv exothermic
Dab tsi yog qhov sib xyaw ntawm cov dej khov thiab cov ntsev ntau hu ua?

Teb: Qhov sib tov no hu ua anti freezing agent. Feem ntau cov lus ntsev yog cov tshuaj sodium chloride
Dab tsi yog qhov txawv ntawm Arrhenius txhais thiab brønsted Lowry txhais ntawm acids thiab bases?
Qhov txawv ntawm peb txoj kev xav yog qhov kev xav Arrhenius hais tias cov kua qaub ib txwm muaj H + thiab cov hauv paus ib txwm muaj OH-. Thaum tus qauv Bronsted-Lowry thov tias cov kua qaub yog cov neeg pub dawb proton thiab cov neeg txais kev lees paub yog li cov hauv paus tsis tas yuav muaj OH- yog li cov kua qaub pub ib qho proton rau dej tsim H3O +
Puas yog NaHS yog ntsev ntsev?

Acid ntsev Cov ntsev tseem muaj hydrogen atom los ntawm cov kua qaub uas tuaj yeem hloov tau los ntawm cov hlau ions ntxiv. Piv txwv xws li: NaHSO4, NaHCO3 thiab NaHS 3. Basic ntsev Cov ntsev muaj cov hydroxides ua ke nrog cov hlau ions thiab cov ions tsis zoo los ntawm cov kua qaub
Puas yog NaH2PO3 yog ntsev ntsev?

Na2HPO3 yog tsim thaum ob lub acidic hydrogens hloov los ntawm sodium. Nws tsis muaj acidic hydrogen. Yog li, nws yog ib txwm sodium ntsev ntawm phosphorus acid. Tab sis NaH2PO3 yog cov ntsev ntsev vim nws tseem muaj ib qho acidic lossis hloov tau hydrogen hauv nws cov muaj pes tsawg leeg
