
Video: Zinc thiab sulfuric acid ua dab tsi?

2024 Tus sau: Miles Stephen | [email protected]. Kawg hloov kho: 2023-12-15 23:36
Zinc reacts nrog sulfuric acid ua form zinc sulphate thiab hydrogen gas yog liberated. Zn + H2SO4 ----> ZnSO4 + H2. Zinc + sulfuric acid --→ zinc sulfate + hydrogen.
Thiab cov lus nug yog, dab tsi ntsev yog zinc thiab sulfuric acid ua?
Sau npe ntsev
| Hydrochloric acid | Sulfuric acid | |
|---|---|---|
| Tooj oxide | Tooj chloride | tooj liab sulfate |
| Aluminium hydroxide | Aluminium chloride | Aluminium sulfate |
| Zinc carbonate | Zinc chloride | Zinc sulfate |
Ib yam li ntawd, yuav ua li cas thaum dilute sulfuric acid rau ntawm zinc phaj? Thaum twg dilute sulfuric acid yog nchuav rau ntawm zinc phaj , Zinc Sulphate yog tsim nrog Hydrogen roj. Peb tuaj yeem kuaj cov pa roj hydrogen los ntawm kev noj cov nplaum nplaum nyob ze ntawm nws, thiab cov roj yuav taws nrog lub suab nrov.
Tsis tas li ntawd, qhov sib npaug ntawm zinc thiab sulfuric acid yog dab tsi?
Zn + H2SO4 = ZnSO4 + H2 | Tshuaj tshuaj tiv thaiv thiab kev sib npaug.
Dab tsi ntsev yog tsim los ntawm sulfuric acid?
Neutralizing sulfuric acid tsim sulfate ntsev. Sulfuric acid + sodium hydroxide → sodium sulfate + dej.
Pom zoo:
Dab tsi yog qhov sib npaug ntawm ammonia thiab sulfuric acid?

Txhawm rau sib npaug NH3 + H2SO4 = (NH4) 2SO4 koj yuav tsum nco ntsoov suav tag nrho cov atoms ntawm txhua sab ntawm cov tshuaj sib npaug
Dab tsi ua rau acid acid thiab ib puag ncig?
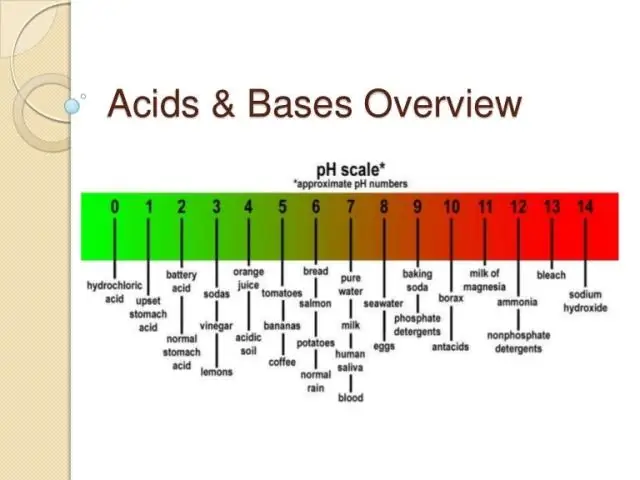
Cov kua qaub yog ib yam khoom uas pub hydrogen ions. Vim li no, thaum cov kua qaub yaj hauv dej, qhov sib npaug ntawm hydrogen ions thiab hydroxide ions hloov. Cov tshuaj no yog acidic. Lub hauv paus yog ib yam khoom uas txais hydrogen ions
Yuav ua li cas tshwm sim thaum dilute sulfuric acid yog nchuav rau ntawm zinc phaj?

Thaum dilute Sulfuric acid yog nchuav rau ntawm ib lub zincplate, Zinc Sulphate yog tsim nrog Hydrogen gas.Peb tuaj yeem kuaj cov roj hydrogen los ntawm kev noj cov khoom siv hluav taws xob ze ze, thiab cov roj yuav ignite nrog lub suab nrov
Qhov loj feem pua ntawm zinc nyob rau hauv zinc II phosphate yog dab tsi?

Cov feem pua ntawm cov ntsiab lus Element Symbol Mass Percent Zinc Zn 50.803% Oxygen O 33.152% Phosphorus P 16.045%
Dab tsi yog qhov sib npaug ntawm tooj liab oxide thiab sulfuric acid?

Txhawm rau sib npaug CuO + H2SO4 = CuSO4 + H2O koj yuav tsum nco ntsoov suav tag nrho cov atoms ntawm txhua sab ntawm cov tshuaj sib npaug. Thaum koj paub ntau npaum li cas ntawm txhua hom atom koj tuaj yeem hloov pauv cov coefficients (tus lej nyob rau hauv pem hauv ntej ntawm atoms lossis cov tebchaw) kom sib npaug ntawm qhov sib npaug rau tooj liab (II) oxide + Sulfuric acid
