
Video: Yuav ua li cas koj xam barium hydroxide?

2024 Tus sau: Miles Stephen | [email protected]. Kawg hloov kho: 2023-12-15 23:36
Barium hydroxide tuaj yeem npaj los ntawm kev tawg barium oxide (BaO) hauv dej: BaO + 9 H2O → Ba(OH)2· 8 H2O. Nws crystallises li octahydrate, uas hloov mus rau monohydrate thaum cua sov hauv huab cua. Ntawm 100 ° C hauv lub tshuab nqus tsev, cov monohydrate yuav tawm los BaO thiab dej.
Hais txog qhov no, tus nqi ntawm barium hydroxide yog dab tsi?
Kev piav qhia: Barium yog ib lub caij hauv Pawg 2, yog li nws muaj ob lub valence electrons, thiab nws yog nqi yog 2+. Hydroxide yog polyatomic tsis zoo ion nrog valency ntawm 1, thiab nws yog nqi yog 1-. Raws li barium yog hlau thaum hydroxide yog cov hlau tsis yog hlau, lawv ua ke los ua ib qho ionic compound.
Ib yam li ntawd, puas barium hydroxide yaj hauv dej? Dej
Ib yam li ntawd, tib neeg nug, puas yog barium hydroxide tsim los nag?
Feem ntau ntawm lub sij hawm, ib tug faint dawb nag xob nag cua ntawm barium hydroxide yog tsim. Barium hydroxide yog me ntsis soluble nyob rau hauv dej thiab muaj peev xwm tsim ib tug tov nrog ib tug concentration ntawm ib ncig ntawm 0.1 M ntawm chav tsev kub; barium hydroxide saum toj no 0.1 M yuav insoluble.
Barium hydroxide dissociate li cas?
(1) Barium hydroxide dissociates tag nrho hauv dej los tsim barium ions thiab hydroxyl ions: {eq}Ba(OH)_2 (s) ightarrow Ba^{2+} (aq) +
Pom zoo:
Dab tsi yog cov khoom lag luam hauv kev sib npaug molecular rau kev ua tiav cov tshuaj tiv thaiv ntawm aqueous barium hydroxide thiab nitric acid?

Ba(OH)2 + 2HNO3 → Ba(NO3)2 + 2H2O. Barium hydroxide react nrog nitric acid los tsim barium nitrate thiab dej
Yuav ua li cas koj xam tus qauv sib txawv ntawm PMP?
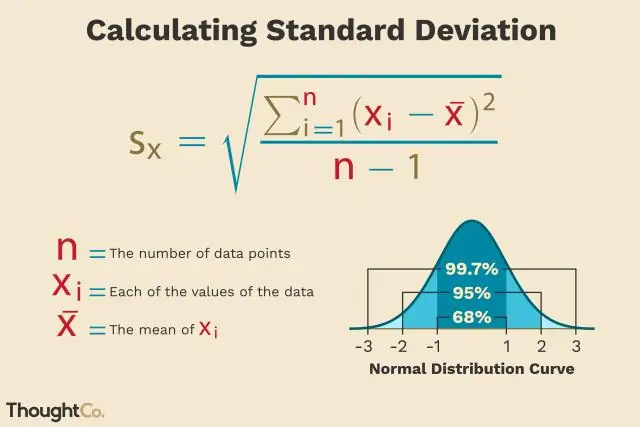
Cov mis siv nyob rau hauv PMBOK rau tus qauv sib txawv yog yooj yim. Nws tsuas yog (P-O) / 6. Qhov ntawd yog qhov pessimistic kev ua ub no kwv yees rho tawm qhov kev kwv yees qhov kev xav tau muab faib los ntawm rau. Qhov teeb meem yog qhov no nyob rau hauv tsis muaj txoj kev zoo los yog daim ntawv tsim ib qho kev ntsuas ntawm tus qauv deviation
Dab tsi yog qhov tseeb ntawm cov ntsev tsim nyob rau hauv cov tshuaj tiv thaiv neutralization ntawm hydrochloric acid nrog barium hydroxide?

Lus Nug: Dab tsi yog cov qauv ntawm cov ntsev tsim nyob rau hauv Neutralization Reaction ntawm Hydrochloric Acid Nrog Barium Hydroxide? BaCl BaCl2 BaClH BaH2 BaO
Yuav ua li cas koj xam lub sij hawm nws yuav siv sij hawm mus ncig teb chaws?

Kwv yees seb koj yuav mus sai npaum li cas ntawm koj qhov kev mus ncig. Tom qab ntawd, faib koj qhov kev ncua deb ntawm koj qhov nrawm. Qhov no yuav muab rau koj anestimation ntawm koj lub sijhawm mus ncig. Piv txwv li, yog tias koj mus ncig yog 240 mais thiab koj yuav tsav 40 mais ib teev, koj lub sijhawm yuav yog 240/40 = 6hours
Yuav ua li cas koj xam lub sij hawm nws yuav siv ib yam khoom los poob?

Ntsuas qhov deb ntawm qhov khoom yuav poob hauv ko taw nrog tus pas ntsuas lossis ntsuas daim kab xev. Faib qhov kev ncua deb los ntawm 16. Piv txwv li, yog tias cov khoom yuav poob 128 ko taw, faib 128 los ntawm 16 kom tau 8. Xam cov square root ntawm cov kauj ruam 2 los nrhiav lub sij hawm nws yuav siv cov khoom poob hauv vib nas this
