Cov txheej txheem:

Video: Cov qauv siv lead ua ntawm cesium chloride yog dab tsi?

2024 Tus sau: Miles Stephen | [email protected]. Kawg hloov kho: 2023-12-15 23:36
Crystal qauv
Cov Cesium chloride qauv adopts primitive cubic daim kab xev nrog ob lub atom hauv paus, qhov twg ob lub atoms muaj yim sib koom ua ke. Cov tshuaj chloride atoms dag rau ntawm daim kab xev cov ntsiab lus ntawm cov npoo ntawm lub voos xwmfab, thaum lub cesium atoms nyob rau hauv lub qhov nyob rau hauv nruab nrab ntawm lub cubes.
Xav txog qhov no, tus qauv ntawm cesium chloride yog dab tsi?
CsCl
Ib yam li ntawd, yog CsCl FCC lossis BCC? Nco ntsoov tias tsis muaj lattice taw tes nyob rau hauv nruab nrab ntawm lub cell, thiab CsCl tsis yog a BCC Cov qauv vim tias cov cesium ion tsis zoo ib yam rau cov chloride ion.
Xav txog qhov no, yog cesium chloride yog BCC?
Crystal qauv Daim duab 3A qhia cov cesium chloride (CsCl) qauv, uas yog ib qho kev npaj cubic. Yog hais tias tag nrho cov atoms nyob rau hauv cov qauv no yog tib hom, nws yog a bcc ua daim kab xev. Cov spheres nyob 68 feem pua ntawm cov ntim. Muaj 23 hlau nrog cov bcc ua kev npaj.
Vim li cas CsCl yog thawj zaug?
CsCl muaj ib daim ntawv cog lus ionic. For form a keeb kwm cubic lattice ob ions yuav tsum muaj qhov sib xws. Cs+ lub vojvoog yog 174 pm thiab Cl- lub vojvoog yog 181 teev tsaus ntuj yog li lawv tsim ib keeb kwm kub latt.
Pom zoo:
Cov qauv siv lead ua ntawm tooj liab sulfate yog dab tsi?

Copper(II) sulfate Names Structure Crystal structure Orthorhombic (anhydrous, chalcocyanite), space group Pnma, oP24, a = 0.839 nm, b = 0.669 nm, c = 0.483 nm. Triclinic (pentahydrate), qhov chaw pab pawg P1, aP22, a = 0.5986 nm, b = 0.6141 nm, c = 1.0736 nm, α = 77.333 °, β = 82.267 °, γ = 72.567 ° Thermochemist
Dab tsi yog atomic packing factor ntawm cov qauv siv lead ua?

Atomic packing factor tseem hu ua packing efficiency ntawm crystal. Nws txhais tau tias yog qhov ntim tau los ntawm kev sib txuas tag nrho cov atoms ntawm ib lub cell hauv kev sib piv rau tag nrho cov ntim ntawm ib chav tsev cell, piv txwv li nws yog ib feem ntawm lub ntim nyob los ntawm tag nrho cov atoms nyob rau hauv ib chav tsev cell mus rau tag nrho cov ntim ntawm ib chav tsev cell
Thaum lub ntim ntawm ib qho piv txwv ntawm cov pa roj yog txo lub siab ntawm cov qauv ntawm cov pa roj?
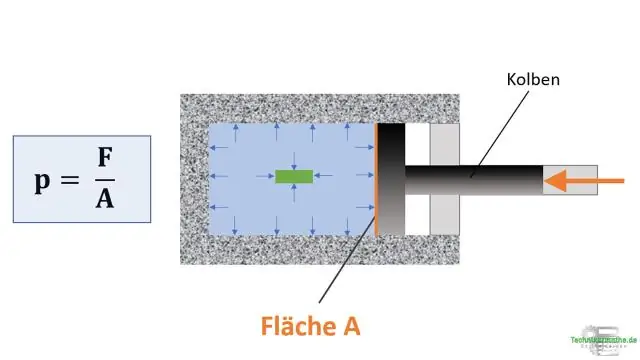
Kev txo qis Cov pa roj sib xyaw txoj cai hais tias lub siab ntawm cov pa roj yog inversely cuam tshuam nrog lub ntim thiab ncaj qha ntsig txog qhov kub thiab txias. Yog tias qhov kub thiab txias yog nyob tas li, qhov sib npaug raug txo rau Boyle txoj cai. Yog li ntawd, yog tias koj txo qhov siab ntawm cov roj av tas li, nws qhov ntim yuav nce ntxiv
Dab tsi yog qhov txawv ntawm cov khoom siv organic thiab cov khoom siv organic?

Dab tsi yog qhov txawv ntawm cov khoom siv organic thiab organic teeb meem? Organic khoom yog txhua yam uas tseem muaj sia nyob thiab tam sim no nyob rau hauv lossis hauv av. Rau nws los ua cov organic teeb meem, nws yuav tsum tau decomposed rau hauv humus. Humus yog cov khoom siv organic uas tau hloov dua siab tshiab los ntawm cov kab mob mus rau lub xeev tsis muaj zog
Tus qauv qauv yog dab tsi Qhov txawv ntawm tus qauv qauv thiab tus qauv molecular yog dab tsi?
Cov mis mos molecular siv cov cim tshuaj thiab cov ntawv sau npe los qhia cov naj npawb ntawm cov atoms sib txawv hauv cov molecule lossis compound. Ib qho empirical formula muab qhov yooj yim tshaj plaws, tag nrho tus lej piv ntawm atoms hauv ib qho chaw. Cov qauv qauv qhia txog kev sib koom ua ke ntawm cov atoms hauv cov molecule
