Cov txheej txheem:

Video: Yuav ua li cas koj xam qhov kub ntawm kev daws?

2024 Tus sau: Miles Stephen | [email protected]. Kawg hloov kho: 2024-01-18 08:15
Heat of Solution los yog Enthalpy of Solution Chemistry Tutorial
- Tus nqi ntawm lub zog tso tawm los yog absorbed yog xam. q = m x cg × ΔT. q = lub zog tso tawm lossis nqus tau.
- xam moles ntawm solute. n = m ÷ M. n = moles of solute.
- Tus nqi zog ( kub ) tso tawm los yog absorbed ib mole ntawm solute yog xam. Δ Hsoln = q ÷ n.
Tsuas yog li ntawd, koj yuav ua li cas xam qhov kub ntawm cov tshuaj hauv kJ mol?
Enthalpy of Solution (Huab cua sov ntawm kev daws) Piv txwv
- Xam cov cua sov tso tawm, q, hauv joules (J), los ntawm cov tshuaj tiv thaiv: q = huab hwm coj (dej) × lub peev xwm kub tshwj xeeb (dej) × hloov pauv hauv qhov kub thiab txias (kev daws teeb meem)
- Xam cov moles ntawm solute (NaOH(s)): moles = mass ÷ molar mass.
- Xam qhov hloov pauv enthalpy, ΔH, hauv kJ mol-1 ntawm solute:
Qhov kub ntawm kev daws ntawm NaOH yog dab tsi? Tus nqi txais rau lub Thaum tshav kub kub ntawm kev daws ntawm NaOH yog 44.2 kJ / mol thiab rau NH4NO3, nws yog 25.4 kJ / mol.
Tom qab ntawd, ib tug kuj yuav nug, ua li cas koj xam cov cua sov absorbed?
Cov kub absorbed yog xam los ntawm kev muab cov moles ntawm dej los ntawm molar kub ntawm vaporization. 5. Chav yog cua sov ntawm 100oC txog 140oC. Cov kub absorbed yog xam los ntawm kev siv qhov tshwj xeeb kub ntawm chav thiab kev sib npaug ΔH=cp × m × ΔT.
Qhov tshwj xeeb cua sov ntawm NaOH yog dab tsi?
Yam tsawg kawg nkaus kub raws li qhov pib kub = 43.5 ° C. Qhov ceev ntawm HCl & NaOH Soultion = 1.04 g/ml. Tshwj xeeb kub ntawm HCl & NaOH Solution = 4.017 J/g°C.
Pom zoo:
Qhov kub ntawm qhov hluav taws kub kub li cas?

Feem ntau hom ntoo yuav pib combusting ntawm li 300 degrees Celsius. Cov pa roj hlawv thiab nce qhov kub ntawm cov ntoo mus txog 600 degrees Celsius (1,112 degrees Fahrenheit). Thaum cov ntoo tso tawm tag nrho nws cov gases, nws tawm charcoal thiab tshauv
Koj yuav daws tau qhov system ntawm peb qhov sib npaug li cas los ntawm kev tshem tawm?

Xaiv ib qho sib txawv ntawm ob qhov sib npaug, hais qhov sib npaug (2) thiab (3), thiab tshem tawm qhov sib txawv. Kev daws qhov system tsim los ntawm kev sib npaug (4) thiab (5). Tam sim no, hloov z = 3 rau hauv kab zauv (4) kom pom y. Siv cov lus teb los ntawm Kauj Ruam 4 thiab hloov mus rau hauv ib qho kev sib npaug uas muaj qhov sib txawv ntxiv
Koj yuav daws tau qhov kub ntawm cov tshuaj tiv thaiv li cas?

Enthalpy of Solution (Heat of Solution) Piv txwv xam cov cua sov tso tawm, q, hauv joules (J), los ntawm cov tshuaj tiv thaiv: q = huab hwm coj (dej) × lub peev xwm kub (dej) × qhov kub thiab txias (kev daws teeb meem) suav cov moles ntawm solute (NaOH(s)): moles = mass ÷ molar mass. Xam qhov hloov pauv enthalpy, ΔH, hauv kJ mol-1 ntawm solute:
Qhov twg yog acidic ntau dua ib qho kev daws ntawm pH 2 lossis kev daws ntawm pH 6?

Kev piav qhia: pH yog qhov ntsuas ntawm acidity lossis alkalinity ntawm cov tshuaj. concentration siab dua yog acidity. Yog li ib qho kev daws ntawm pH = 2 yog acidic ntau dua li ntawm pH = 6 los ntawm qhov zoo ntawm 10000
Koj yuav daws qhov sib npaug li cas los ntawm kev cais qhov sib txawv?
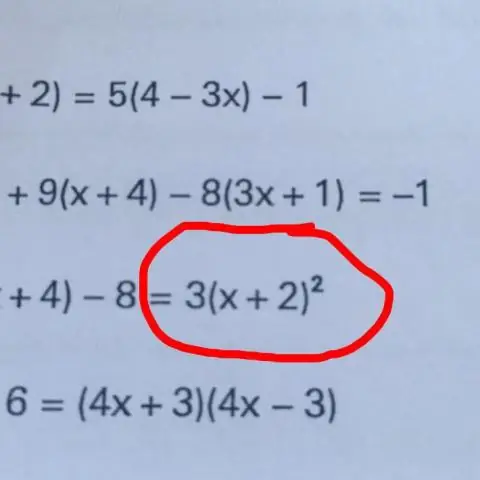
Cov txheej txheem yooj yim los cais qhov sib txawv yog "ua ib yam dab tsi rau ob tog" ntawm qhov sib npaug, xws li ntxiv, rho tawm, sib npaug, lossis faib ob sab ntawm qhov sib npaug los ntawm tib tus lej. Los ntawm kev rov ua cov txheej txheem no, peb tuaj yeem tau txais qhov sib txawv ntawm ib sab ntawm qhov sib npaug
