
Video: Qhov Valency ntawm carbon thiab oxygen yog dab tsi?

2024 Tus sau: Miles Stephen | [email protected]. Kawg hloov kho: 2023-12-15 23:36
Raws li nyob rau hauv carbon dioxide molecule ob carbon zoo li oxygen tau ua tiav lawv cov octet bysharing electrons. #Ib leeg carbon muaj 4 valency thiab oxygen muaj 2. Nws yog + ntxiv 4. Raws li nws yog bounded los ntawm 2 oxygen atoms uas muaj -2 nqi ntawm txhua.
Tsis tas li ntawd, Valency ntawm carbon yog dab tsi?
Tetra- valency ntawm cov pa roj carbon muaj tus lej atomic ntawm 6, lub ntsiab lus txhua carbon atom muaj tag nrho ntawm 6 electrons. Ob tug yog nyob rau hauv lub tiav puab orbit, thaum plaub electrons pom nyob rau hauv lub atom lub sab hnub poob orbit.
Ib yam li ntawd, vim li cas Valency ntawm carbon yog 2? Teb: ( 2 ) raws li plaub valence electrons ntawm txhua carbon atom los ntawm nws lub plhaub sab nraud yog koom nrog covalent bonding soits valency yog 4 (Tetravalent). valency rau carbon yuav nyob twj ywm tib yam li 4. vim nws yog sib koom nwsselectrons.
Ntawm no, dab tsi yog qhov Valency ntawm oxygen?
Cov valency ntawm oxygen yog -2. Qhov no txhais tau tias oxygen yuav tsum tau nce los yog faib ob electrons forstability.
Vim li cas carbon Valency 4?
Vim a carbon atom muaj plaub electrons nyob rau hauv nws sab nraud valence plhaub. Yog li, nws xav tau plaub lub electrons ntxiv kom ua tiav nws cov octet. A carbon atom ua kom tiav nws octet tsuas yog sib qhia nws valence electrons nrog lwm cov atoms. Raws li qhov tshwm sim, a carbon atom tsim plaub covalent bonds los ntawm kev sib koom valence electrons nrog lwm cov atoms.
Pom zoo:
Dab tsi yog qhov txawv ntawm qhov pom tseeb qhov loj thiab qhov ntsuas qhov ntsuas qhov tseeb?

Dab tsi yog qhov txawv ntawm qhov pom tseeb thiab qhov tseeb? Pom meej qhov loj npaum li cas yog qhov ci ntawm lub hnub qub tshwm los ntawm lub ntiaj teb thiab nyob ntawm qhov ci thiab qhov deb ntawm lub hnub qub. Qhov loj npaum li cas yog qhov ci ntawm lub hnub qub yuav tshwm sim los ntawm tus qauv nyob deb
Dab tsi yog qhov txawv ntawm qhov sib txawv ntawm qhov sib txawv thiab qhov sib txawv ntawm qhov ntab?

Integers thiab floats yog ob yam sib txawv ntawm cov ntaub ntawv tus lej. Ib tus lej (feem ntau hu ua anint) yog tus lej tsis muaj tus lej lej. Float isa floating-point number, uas txhais tau hais tias nws yog tus lej uas muaj tus lej lej. Floats siv thaum xav tau ntau dua
Qhov txawv ntawm qhov loj ntawm ib tug proton thiab qhov loj ntawm ib tug electron yog dab tsi?
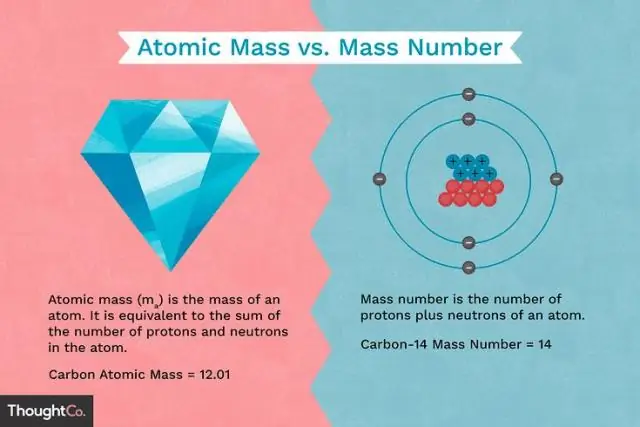
Protons thiab neutrons muaj kwv yees li qub, tab sis lawv yog ob qho tib si loj dua li cov hluav taws xob (kwv yees li 2,000 npaug loj li electron). Tus nqi zoo ntawm ib qho proton yog sib npaug hauv qhov loj rau qhov tsis zoo ntawm tus electron
Dab tsi yog qhov txawv ntawm instantaneous thiab nruab nrab ceev dab tsi yog qhov piv txwv loj tshaj ntawm kev ceev ceev?
Qhov nruab nrab ceev yog qhov ceev nruab nrab ntawm lub sijhawm. Instantaneous ceev yuav yog qhov ceev txhua qhov muab tam sim ntawd hauv lub sijhawm ntawd, ntsuas nrog lub ntsuas ntsuas lub sijhawm
Dab tsi yog qhov txawv ntawm microevolution thiab macroevolution Dab tsi yog qee qhov piv txwv ntawm txhua tus?

Microevolution vs. Macroevolution. Piv txwv ntawm cov kev hloov microevolutionary no yuav suav nrog kev hloov ntawm hom xim lossis loj. Macroevolution, piv txwv li, yog siv los hais txog kev hloov pauv ntawm cov kab mob uas tseem ceeb txaus uas, dhau sijhawm, cov kab mob tshiab yuav raug suav tias yog hom tshiab nkaus xwb
