
Video: Daim duab Bohr txhais li cas?
2024 Tus sau: Miles Stephen | [email protected]. Kawg hloov kho: 2023-12-15 23:36
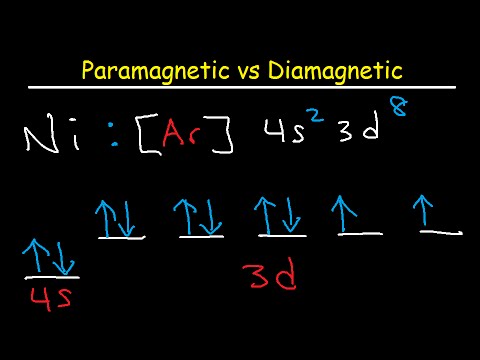
Daim duab Bohr . Bohr daim duab qhia cov electrons orbiting lub nucleus ntawm ib lub atom zoo li cov ntiaj chaw orbit ncig lub hnub. Hauv Bohr qauv , electrons yog pictured raws li kev mus ncig hauv lub voj voog ntawm cov plhaub sib txawv, nyob ntawm seb lub caij twg koj muaj. Txhua lub plhaub ua tau tsuas tuav qee tus lej ntawm electrons.
Yog li ntawd, daim duab Bohr yog dab tsi?
Daim duab Bohr yog ib qho yooj yim pom kev sawv cev ntawm lub atom uas yog tsim los ntawm Danish physicist Niels Bohr nyob rau hauv 1913. Daim duab qhia txog lub atom raws li ib tug zoo nqi. nucleus nyob ib puag ncig electrons uas taug kev nyob rau hauv ncig orbits txog lub nucleus nyob rau hauv discrete zog theem.
Qhov thib ob, tus qauv Bohr tau txais txoj cai li cas? Xyoo 1913, Bohr qhia tias electrons tsuas tuaj yeem muaj qee yam kev txav mus los: Electrons hauv atoms orbit lub nucleus. Cov electrons tuaj yeem tsuas yog orbit ruaj khov, tsis muaj hluav taws xob, hauv qee qhov orbits (hu ua Bohr lub "stationary orbits") ntawm ib qho kev sib cais ntawm qhov deb ntawm lub nucleus.
Cov lus nug tseem yog, Bohr qauv piav qhia li cas?
Bohr Atomic Qauv : Xyoo 1913 Bohr npaj nws lub plhaub quantized qauv ntawm atom rau piav yuav ua li cas electrons tuaj yeem muaj qhov chaw ruaj khov nyob ib ncig ntawm lub nucleus. Lub zog ntawm ib qho hluav taws xob nyob ntawm qhov loj ntawm lub orbit thiab qis dua rau cov orbits me. Radiation tuaj yeem tshwm sim tsuas yog thaum lub electron dhia ntawm ib lub orbit mus rau lwm qhov.
Yuav ua li cas kos ib daim duab Bohr?
- Kos lub nucleus.
- Sau tus naj npawb ntawm neutrons thiab tus naj npawb ntawm protons hauv lub nucleus.
- Kos thawj qib zog.
- Kos cov electrons hauv qib zog raws li cov cai hauv qab no.
- Nco ntsoov tias muaj pes tsawg tus electrons muab tso rau hauv txhua qib thiab pes tsawg tus electrons tshuav siv.
Pom zoo:
Vim li cas nws thiaj li hais tias lub Orthocenter ntawm daim duab peb sab obtuse yuav tsum nyob rau sab nraud ntawm daim duab peb sab?

Nws hloov tawm tias tag nrho peb qhov siab ib txwm sib tshuam ntawm tib lub ntsiab lus - lub npe hu ua orthocenter ntawm daim duab peb sab. Lub orthocenter tsis yog ib txwm nyob hauv daim duab peb sab. Yog tias daim duab peb sab yog obtuse, nws yuav nyob sab nraud. Txhawm rau ua kom qhov no tshwm sim cov kab qhov siab yuav tsum tau txuas ntxiv kom lawv hla
Dab tsi yog qhov txawv ntawm daim duab thiab daim duab?

Ib daim duab yog ib daim duab ntawm kev ua lej, tab sis kuj siv tau (loos) txog ib daim duab ntawm cov ntaub ntawv txheeb cais.A daim duab yog ib daim duab sawv cev ntawm cov ntaub ntawv, qhov twg ib kab ntawv yog ib daim ntawv
Qhov kev hloov pauv twg yuav hloov daim duab A rau hauv daim duab B?
Ob daim duab tau hais tias yuav sib haum xeeb yog tias ib tus tuaj yeem tau txais los ntawm lwm tus los ntawm ib ntu ntawm kev txhais lus, kev xav, thiab kev sib hloov. Cov duab sib xws muaj qhov loj thiab zoo tib yam. Txhawm rau hloov daim duab A rau hauv daim duab B, koj yuav tsum xav txog nws hla y-axis thiab txhais ib chav nyob rau sab laug
Dab tsi yog qhov txawv ntawm lub xeev daim duab kos duab thiab daim duab ua haujlwm?

Lub xeev daim duab qauv yog siv los qhia qhov sib lawv liag ntawm lub xeev uas ib yam khoom mus dhau, qhov ua rau kev hloov ntawm ib lub xeev mus rau lwm qhov thiab qhov kev txiav txim uas tshwm sim los ntawm lub xeev hloov. Daim duab ua haujlwm yog ntws ntawm kev ua haujlwm yam tsis muaj qhov tshwm sim (kev tshwm sim) mechanism, lub xeev tshuab yog suav nrog lub xeev triggered
Txhais tau li cas los txhais ib daim duab peb sab?

Kev txhais lus. Kev txhais lus yog ib qho kev hloov pauv uas tshwm sim thaum ib daim duab tau txav los ntawm ib qho chaw mus rau lwm qhov chaw yam tsis tau hloov nws qhov loj, duab lossis kev taw qhia. Txhawm rau txhais cov ntsiab lus P (x, y), ib chav nyob sab xis thiab b units, siv P'(x + a, y + b)
