
Video: Koj pom H+ los ntawm HCL li cas?

2024 Tus sau: Miles Stephen | [email protected]. Kawg hloov kho: 2023-12-15 23:36
Hauv qhov piv txwv, ib qho molecule ntawm HCl tsim ib hydrogen ion. Muab cov kua qaub ntau ntau los ntawm tus naj npawb ntawm hydrogen ions tsim los suav cov concentration [H +]. Piv txwv li, yog hais tias lub concentration ntawm HCL Hauv cov tshuaj yog 0.02 molar, ces qhov concentration ntawm hydrogen ions yog 0.02 x 1 = 0.02 molar.
Tom qab ntawd, dab tsi yog H + ntawm HCl?
Ua cov kua qaub uas muaj zog, peb tuaj yeem xav tias HCl tag nrho dissociates (ionizes) hauv dej. Tsis tas li ntawd, txij li ib qho molecule ntawm HCl yields ib [ H+ ], qhov sib npaug yog sib npaug rau cov molecular mass. Yog li ntawd ib tug molar tov ntawm HCl (ib molecular mass ib liter), yields ib molar tov ntawm [ H+ ].
Ib tug kuj yuav nug, ua li cas koj xam H + los ntawm pH? Cov pH ntawm ib qho kev daws yog sib npaug rau lub hauv paus 10 logarithm ntawm lub H+ concentration, muab faib ua -1. Yog koj paub cov pH ntawm kev daws dej, koj tuaj yeem siv qhov no qauv rov qab mus nrhiav cov antilogarithm thiab xam tus H+ concentration nyob rau hauv qhov kev daws teeb meem. Cov kws tshawb fawb siv pH ntsuas seb cov dej acidic lossis cov dej yooj yim npaum li cas.
Hauv no, puas yog H + Tib yam li HCl?
Hydrochloric acid ( HCl ) faib ua Hydrogen Ions (H +) thiab Chloride Ions (Cl-). Ntxiv H+ txhais tau tias kua qaub (tsis muaj qhov sib npaug ntxiv).
Puas yog HCl muaj zog acid?
A muaj zog acid yog ib kua qaub uas yog kiag li ionized nyob rau hauv ib tug aqueous tov. Hydrogen chloride ( HCl ) ionizes tag nrho rau hauv hydrogen ions thiab chloride ions hauv dej. Ib qho tsis muaj zog kua qaub yog ib kua qaub uas ionizes tsuas yog me ntsis hauv cov tshuaj aqueous. Vim HCl yog a muaj zog acid , nws conjugate puag (Cl−) yog qaug zog heev.
Pom zoo:
Yuav ua li cas koj pom joules los ntawm wavelength?

Qhov sib npaug rau kev txiav txim siab lub zog ntawm photon ofelectromagnetic hluav taws xob yog E = h ν qhov twg E yog lub zog hauv Joules, h yog Planck qhov tas li, 6.626 × 10 − 34J ν (pronounced 'noo') yog qhov zaus. Koj tau txais lub wavelength λ (hais lambda) hauv nanometers, tab sis tsis yog qhov zaus
Koj pom molarity li cas los ntawm qhov ntom thiab feem pua?
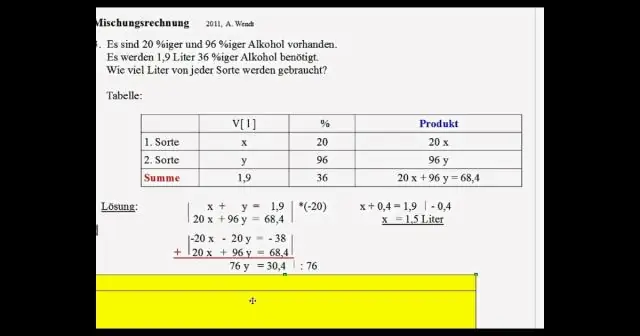
Molarity yog tus naj npawb ntawm moles ntawm soluteper liter ntawm kev daws. Hloov mus rau qhov ntom los ntawm kev muab cov lej ntawm moles los ntawm cov molecular loj ntawm cov compound.Hloov qhov ntom rau molarity los ntawm kev hloov mus rau gramsper liter thiab faib los ntawm molecular mass ntawm cov compound ingrams
Yuav ua li cas koj pom molarity los ntawm absorbance?

Qhov sib npaug yuav tsum yog y = mx + b daim ntawv. Yog li yog tias koj rho koj y-intercept los ntawm absorbance thiab faib los ntawm txoj kab nqes, koj tab tom nrhiav qhov concentration ntawm koj tus qauv
Yuav ua li cas koj pom qhov concentration ntawm DNA los ntawm absorbance?

DNA concentration yog kwv yees los ntawm kev ntsuas qhov nqus ntawm 260nm, kho qhov ntsuas A260 rau turbidity (tseem los ntawm absorbance ntawm 320nm), muab faib los ntawm cov khoom dilution, thiab siv kev sib raug zoo uas A260 ntawm 1.0 = 50µg / ml ntshiab dsDNA
Yuav ua li cas koj pom tus qauv enthalpy ntawm tsim los ntawm combustion?

Tus qauv enthalpy ntawm cov tshuaj tiv thaiv (Δ Horxn) tuaj yeem xam los ntawm cov lej ntawm cov qauv enthalpies ntawm kev tsim cov khoom (txhua qhov sib npaug los ntawm nws cov stoichiometric coefficient) rho tawm cov lej ntawm cov qauv enthalpies ntawm kev tsim cov reactants (txhua qhov sib npaug los ntawm nws. stoichiometric coefficient) - "cov khoom
