Cov txheej txheem:

Video: Koj pom lattice zog li cas?

2024 Tus sau: Miles Stephen | stephen@answers-science.com. Kawg hloov kho: 2024-01-18 08:15
Ntsiab Lus
- Lattice zog yog txhais raws li tus zog yuav tsum cais ib mole ntawm ib qho khoom ionic rau hauv gaseous ions.
- Lattice zog tsis tuaj yeem ntsuas qhov pom, tab sis nws tuaj yeem suav nrog siv electrostatics lossis kwv yees siv lub voj voog yug-Haber.
Ib yam li ntawd, tib neeg nug, dab tsi yog lattice zog mis?
Lattice Zog thiab lub zog ntawm IonicBond Kev kwv yees ntawm lub zog ntawm cov ntawv cog lus hauv ib qho ioniccompound tuaj yeem tau los ntawm kev ntsuas qhov lattice zog ntawm lub compound, uas yog zog muab tawm thaum oppositelycharged ions nyob rau hauv cov roj theem tuaj ua ke los tsim assolid.
Ib tug kuj yuav nug, yog Lattice Zog ib txwm tsis zoo? Lattice Zog . Lwm lub ntsiab txhais hais tias lattice zog yog tus txheej txheem thim rov qab, lub ntsiab lus nws yog tus zog tso tawm thaum gaseous ions khi los ua ib qho ionicsolid. Raws li implied nyob rau hauv lub ntsiab txhais, cov txheej txheem no yuav ib txwm yuav exothermic, thiab yog li tus nqi rau latticeenergy yuav yog tsis zoo.
Ib yam li ntawd, tib neeg nug, qhov tsis tu ncua k hauv lattice zog yog dab tsi?
Tus nqi ntawm cov tas k ' nyob ntawm qhov kev npaj tshwj xeeb ntawm ions hauv cov khoom daim kab xev thiab lawvvalence electron configurations. Sawv cev qhov tseem ceeb forcalculated lattice zog , uas muaj li ntawm 600 mus rau 10, 000 kJ / mol, tau teev nyob rau hauv Table 1.
Qhov twg muaj ntau dua lattice zog NaCl lossis MgCl2?
U( MgCl 2 ) = 2477; U( NaCl ) = 769 kJ mol^-1 Ntau dua lattice zog implies zoo stability lub ntsiab lus muaj zog bonds.
Pom zoo:
Koj ua li cas xam lub hwj chim tiag tiag thiab lub zog pom tseeb?
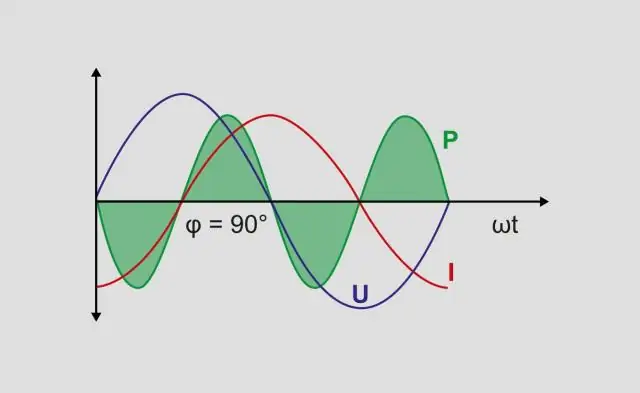
Kev sib xyaw ua ke ntawm lub zog hluav taws xob thiab lub zog muaj tseeb yog hu ua lub zog pom tseeb, thiab nws yog cov khoom lag luam ntawm lub zog hluav taws xob thiab tam sim no, tsis hais txog theem kaum. Lub zog pom meej yog ntsuas hauv chav tsev ntawm Volt-Amps (VA) thiab yog cim los ntawm tsab ntawv loj S
Koj pom qhov hloov pauv hauv lub zog ntawm cov pa roj li cas?

Hloov hauv lub zog sab hauv ntawm cov pa roj ntawm qhov ntim tas li. Raws li thawj txoj cai ntawm thermodynamics, u = q + w, qhov twg koj hloov pauv hauv lub zog sab hauv, q yog cov cua sov liberated thiab w yog cov haujlwm ua tiav hauv cov txheej txheem. Tam sim no ntawm qhov ntim tas li, w = 0, li no u = q
Koj pom li cas pH ntawm qhov sib npaug ntawm cov kua qaub thiab lub hauv paus muaj zog?

Ntawm qhov sib npaug, qhov sib npaug ntawm H + thiab OH- ions yuav sib xyaw ua H2O, ua rau pH ntawm 7.0 (tsis nruab nrab). Lub pH ntawm qhov sib npaug ntawm qhov sib npaug ntawm qhov titration yuav ib txwm yog 7.0, nco ntsoov tias qhov no tsuas yog muaj tseeb rau titration ntawm cov kua qaub muaj zog nrog lub hauv paus muaj zog
Vim li cas lattice zog txo nrog qhov loj?

Raws li lub vojvoog ntawm ions nce, lub lattice zog txo qis. Qhov no yog vim hais tias nrog kev loj hlob ntawm cov noob qoob loo, qhov kev ncua deb ntawm lawv cov nuclei nce. Yog li theattraction ntawm lawv txo qis thiab thaum kawg lub lesslattice zog tso tawm thaum tus txheej txheem
Yuav ua li cas koj pom qhov zaus ntawm qhov ua kom muaj zog?

Qhov sib npaug Arrhenius yog k = Ae^(-Ea / RT), qhov twgA yog qhov zaus lossis qhov ua ntej exponential thiabe^(-Ea / RT) yog cov feem ntawm kev sib tsoo uas muaj lub zog txaus los teb (xws li, muaj zog ntau dua orequal. mus rau activation zog Ea) ntawm kub T
