
Video: Koj sau PbO li cas?

2024 Tus sau: Miles Stephen | [email protected]. Kawg hloov kho: 2023-12-15 23:36
Kev piav qhia ntawm yuav ua li cas sau lub npe rau PbO , Lead (II) oxide. Ua ntej peb txiav txim siab seb PbO yog ib qho ionic los yog molecular (covalent) compound siv lub sij hawm ntev. Los ntawm lub sij hawm lub sij hawm Pb yog hlau thiab O yog nonmetal. Yog li ntawd PbO yog ib qho ionic compound vim nws muaj hlau thiab nonmetal.
Tsuas yog li ntawd, tus nqi ntawm PbO yog dab tsi?
Pb has zero nqi thiab O muaj -2 nqi . Thaum koj tig lub nqi Pb tam sim no muaj qhov zoo 2 thiab O muaj xoom nqi . Qhov no yog vim li cas nws tawm los ua Lead (II) oxide.
Ib sab saum toj no, hom ntawv cog lus dab tsi yog PbO? Hauv Pb3O4 cov pa oxygen octahedra muaj Pb4+ ions, txhua tus uas pub nws plaub sab nraud electrons rau cov pa oxygens nyob ib puag ncig octahedron, thiab yog li tsim cov ionic feem ntau. daim ntawv cog lus.
Yog li, PbO oxidation lub xeev yog dab tsi?
+4
Puas yog PbO ionic lossis covalent?
Raws li cov lus teb tseem ceeb, PbO tsis yog ionic . Txawm tias kuv paub qhov txawv ntawm no ionic thiab covalent tsis tau txhais kom meej. Tab sis Pb yog hlau thiab Oxygen tsis yog hlau.
Pom zoo:
Yuav ua li cas koj sau cov mis rau ib tug compound uas muaj ib tug polyatomic ion?
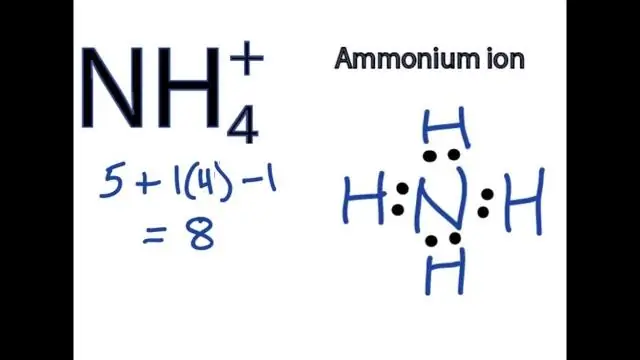
Txhawm rau sau cov qauv rau cov tebchaw uas muaj polyatomic ions, sau lub cim rau cov hlau ion ua raws li cov qauv rau polyatomic ion thiab sib npaug cov nqi. Txhawm rau sau npe ib qho chaw uas muaj polyatomic ion, hais cov cation ua ntej thiab tom qab ntawd cov anion
Yuav ua li cas sau ib lub dab dej hauv koj lub vaj?

Sau lub sinkhole nrog ob peb nti ntawm av. Siv ib tug hlau bar los yog sab saum toj ntawm ib tug sledgehammer los ntim cov av kom ruaj khov rau hauv lub qhov. Txuas ntxiv sau lub qhov nrog av thiab khov kho nws kom txog rau thaum koj mus txog rau sab saum toj ntawm lub dab dej. Nyob rau saum npoo, siv ib txhais tes tamper ntim cov topsoil nyob rau hauv qhov chaw
Yuav ua li cas koj sau ib qho kev sib npaug hauv point slope form muab ob lub ntsiab lus?

Muaj ntau hom ntawv uas peb tuaj yeem sau qhov sib npaug ntawm ib kab: daim ntawv point-slope, daim ntawv nqes hav-intercept form, tus qauv daim ntawv, thiab lwm yam. Qhov sib npaug ntawm ib kab muab ob lub ntsiab lus (x1, y1) thiab (x2, y2). ) los ntawm cov kab dhau los yog muab los ntawm, ((y - y1) / (x - x1)) / ((y2 - y1) / (x2 - x1))
Koj sau Ap Lang sau ntawv li cas?

Synthesis Writing Dos and Don'ts DO Tsim kom muaj zog, Clear Thesis Statement. TSIS siv cov kab lus. Cite koj cov peev txheej kom raug thiab tsim nyog. DO Sketch a Basic Outline. UA Pace Koj tus kheej. Ua pov thawj thiab kho koj cov ntawv sau ua tib zoo
Koj puas siv cov lus qhia hauv kev sau ntawv sau?

Tsab ntawv no yog ntsuas koj lub peev xwm los tsim qhov kev sib cav uas siv cov peev txheej. Yog tias koj siv cov ntaub ntawv los ntawm cov peev txheej, koj yuav tsum sau nws. Yog tias koj nqa cov ntawv tawm ntawm qhov chaw, koj yuav tsum muab tso rau hauv cov lus hais. Tom qab koj hais los yog piav ib qhov chaw, hais nws raws li koj xav tau hauv ib daim ntawv: (Source F) lossis (Gilman)
